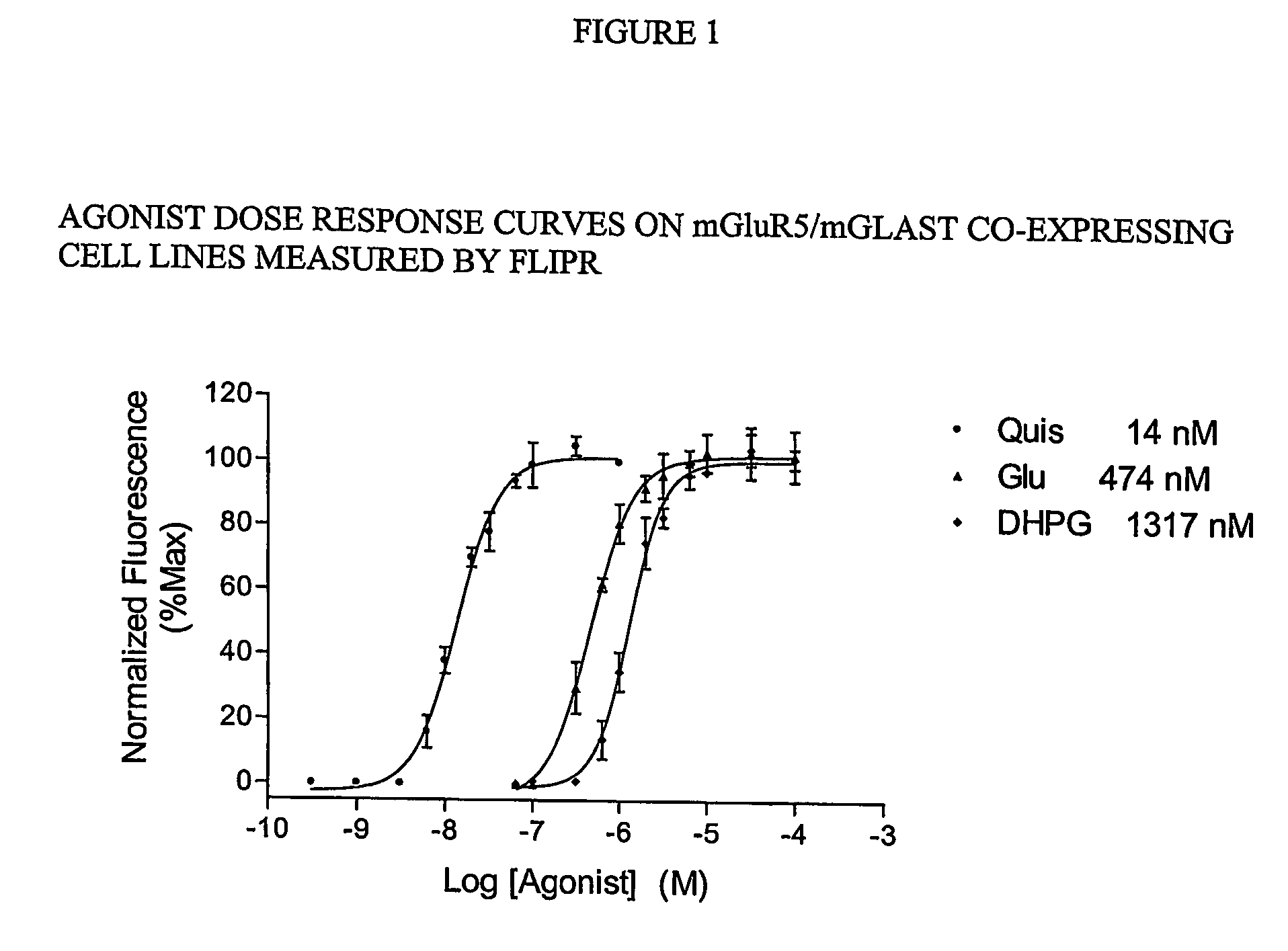
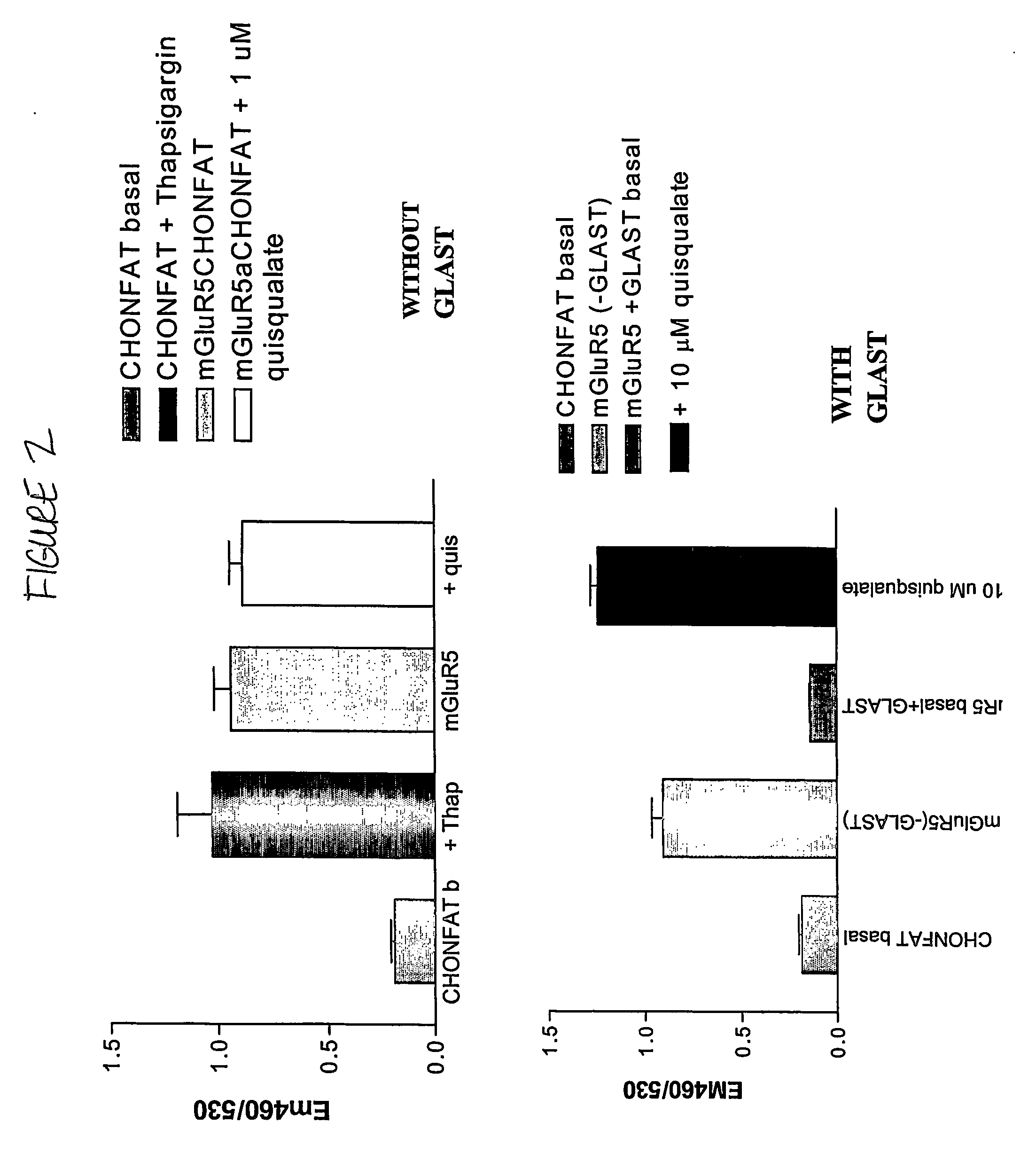
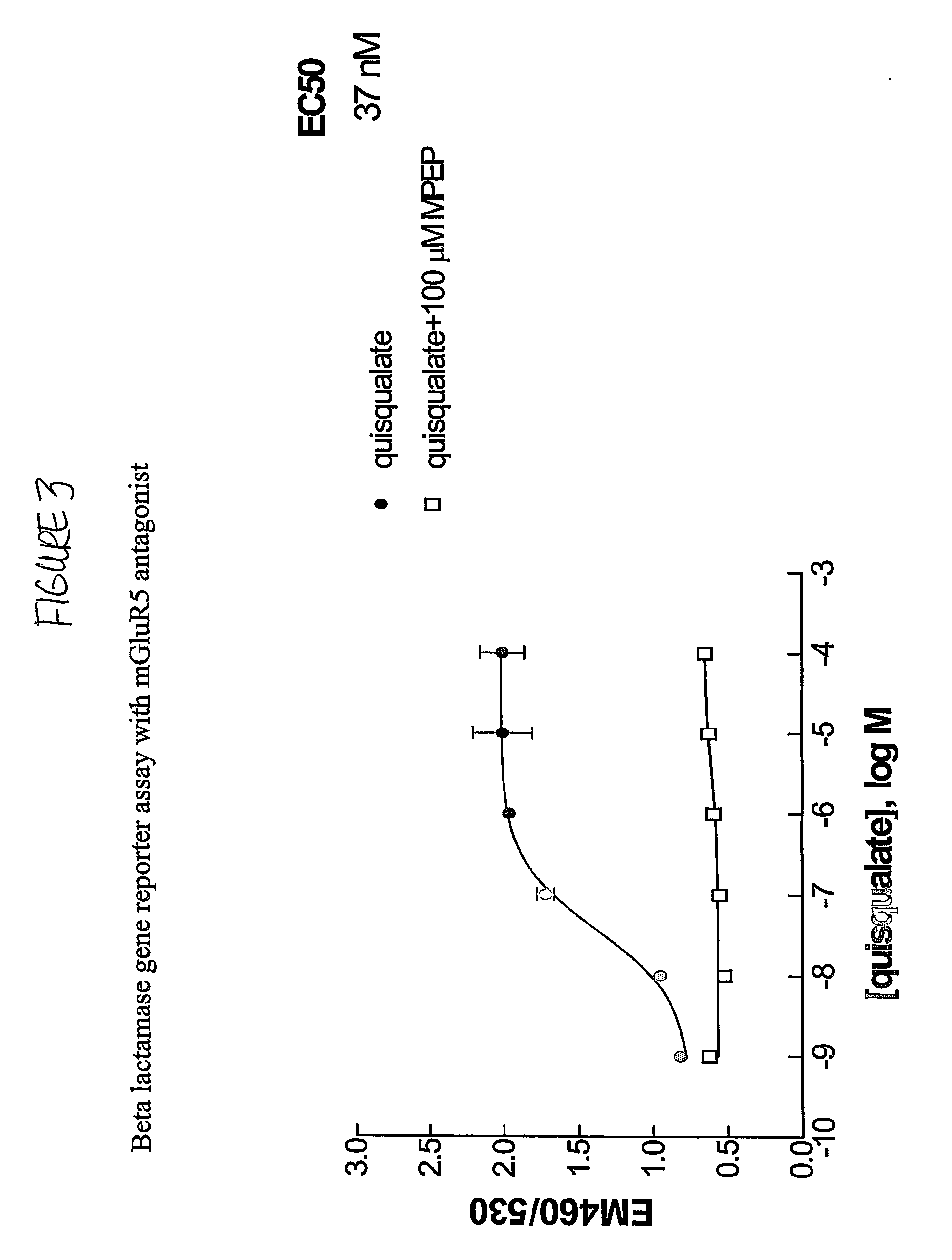
Methods for identifying cell surface receptor protein modulators
a cell surface receptor and protein technology, applied in the field of methods for identifying cell surface receptor protein modulators, can solve the problems of changing ion permeability and electrical potential in the postsynaptic cell, the limited use of currently available mglur agonists and antagonists, and the generation of data by conventional high-throughput systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
CLONING OF mGLAST
[0271] The full-length cDNA of mouse GLAST was isolated by PCR from a Marathon-Ready mouse brain cDNA library (Clontech, Palo Alto, CA). A 1725 bp fragment was amplified by PCR using Pfu Turbo DNA Polymerase with the following cycling conditions: a 2 min pre-incubation at 95° C., followed by 35 cycles of 95° C. for 30 sec., 56° C. for 30 sec., and 72° C. for 3 min. This fragment was obtained using the N-terminal primer, (5′GCCACCATGACCAAAAGCAACGGAGA 3′) containing an optimized Kozak sequence (GCCACC) and the C-terminal primer (5′ GAAAGTGAGCCCAGGGAGAT 3′) resulting in the inclusion of 80 basepairs of 3′ untranslated region. The amplified fragment was cloned into the PCR-Blunt II-Topo vector (Invitrogen, Carlsbad, Calif.). Following confirmation of the DNA sequence, the entire coding sequence of the gene was excised by EcoRI and sub-cloned into the mammalian expression vector pIRESneo2 (Invitrogen, Carlsbad, Calif.).
Generation of Stable Cell Lines Co-Eexpressing m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lag time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com