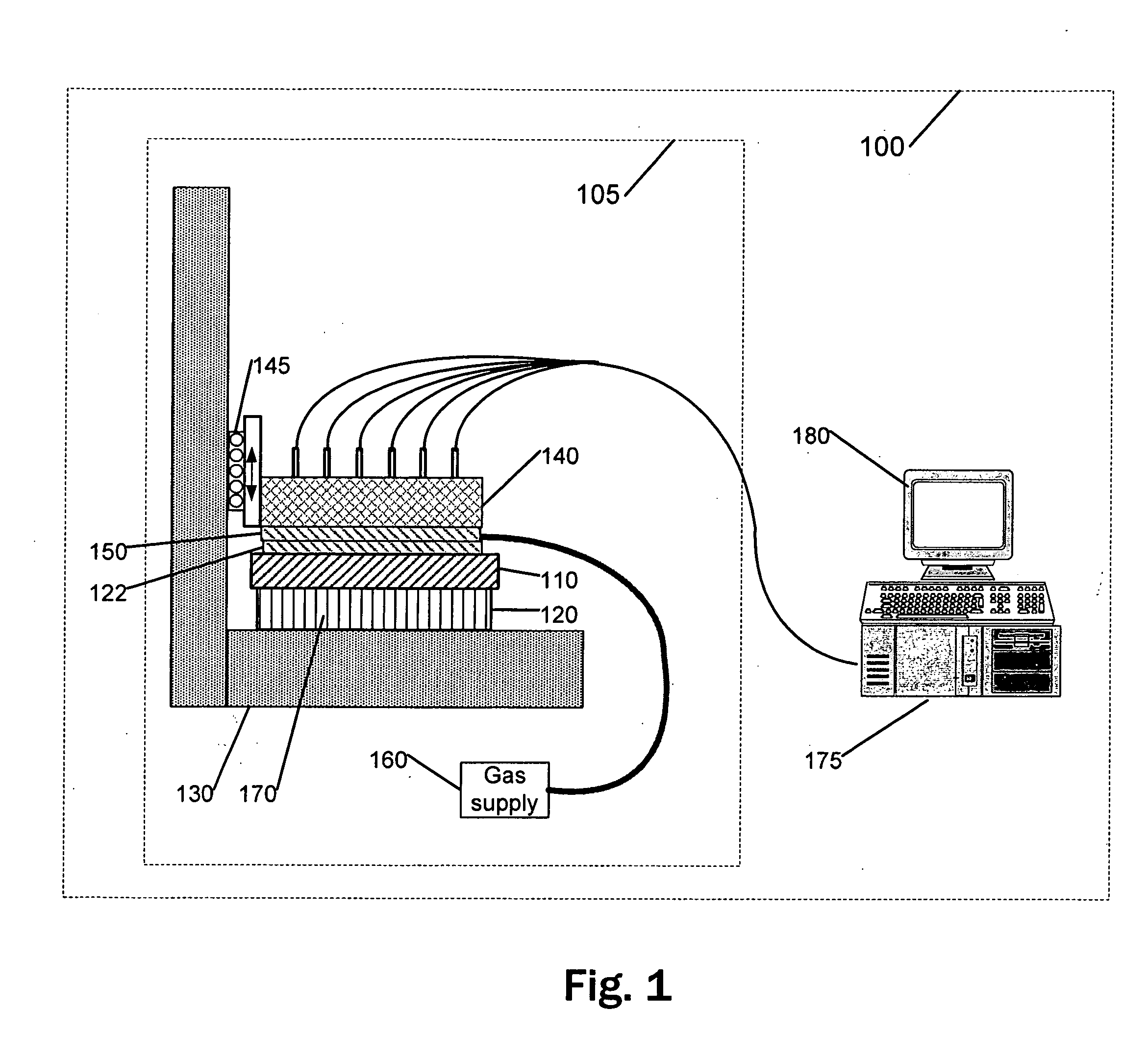
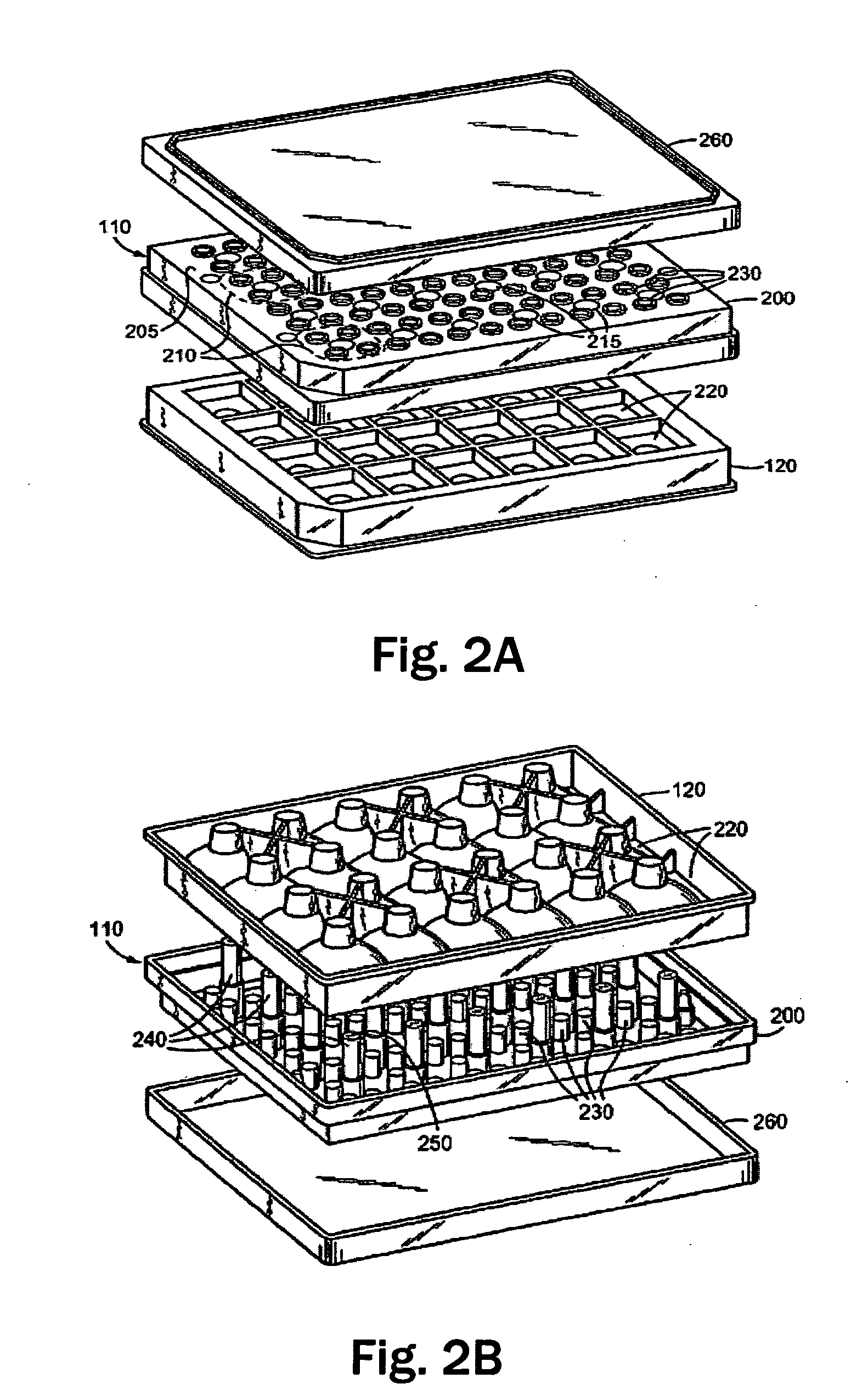
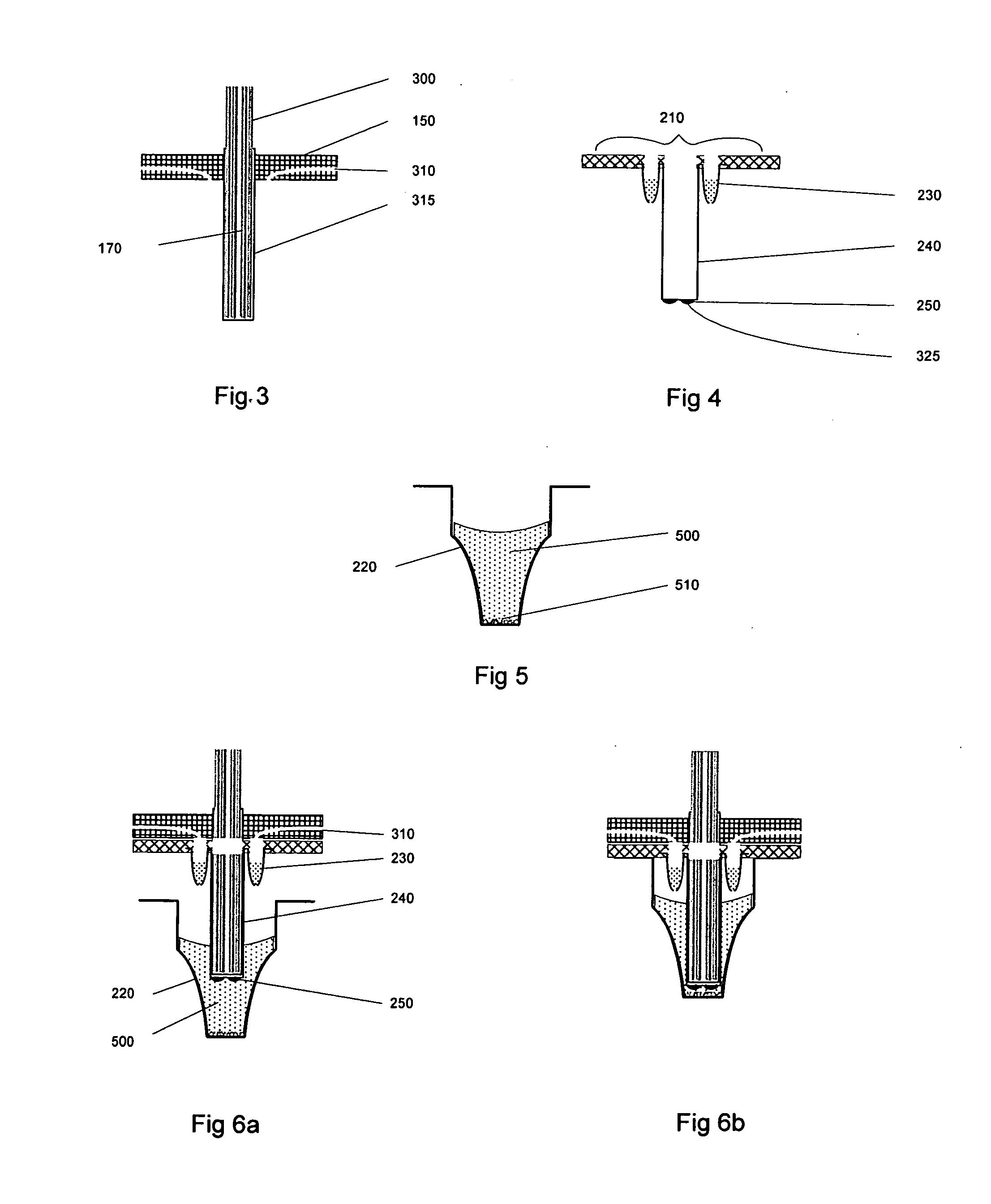
Analysis of metabolic activity in cells using extracellular flux rate measurements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Real-Time Measurement of Changes in Cellular Energetic Pathways in LNCaP Cells in Response to Metabolic Modulators
[0136] Cancer cells were exposed to a series of drugs that impact metabolic function in order to determine the ability of the extracellular flux (XF) assay to interrogate changes in metabolic function. Prostate cell line LNCaP was obtained from the ATCC (Manassas, Va.). LNCaP cells were maintained in modified RPMI 1640 media supplemented with 10% fetal bovine serum (FBS) and 100 μg / ml penicillin-Streptomycin. A highly invasive and metastative, in vivo-derivative of LNCaP, C4-2 cells were maintained in T medium. 2,4-DNP, 2-deoxyglucose, myxothiazol and Calcein AM were prepared according to the manufacturer's instructions. Metabolic flux rate measurements were performed using a prototype Seahorse XF instrument as described above. This instrument was configured to measure the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), corresponding to proton...
example 2
H460 Cells Exhibit Attenuated Mitochondrial Respiratory Capacity as Compared to A549 Cells
[0145] Experiments were performed as disclosed above to demonstrate and confirm the increased glycolytic capacity and more aberrant mitochondrial respiration in a highly metastatic cancer cell line.
[0146] As shown in FIG. 13, a maximal increase of 150% in OCR over baseline was observed in H460 cells in response to glycolysis inhibitors (A), oxamate, 3-bromopyruvic acid, iodoacetate and fluoride. Since the maximum OCR increase following treatment with the mitochondrial uncoupler 2,4-DNP was also 150%, this suggests that lower mitochondrial respiratory capacity is an intrinsic characteristic of H460 cells (B). In comparison, maximal 225% and 250% responses were observed in A549 cells following glycolysis inhibitors or 2,4-DNP respectively.
example 3
Doxorubicin Mediated Cytotoxicity in H460 Cells
[0147] XF assays were used to profile the dose response of the chemotherapeutic compound Doxorubicin. H460 cancer cells were exposed to various doses of Doxorubicin for 72 hours, then profiled using XF assays as described above. As shown in FIG. 14, a biphasic OCR response was measured in which oxygen consumption was enhanced at doses below 10 nM and inhibited at doses exceeding 10 nM, suggesting stimulation of aerobic metabolism to support increased ATP demands of the cell at doses below the highly cytotoxic concentrations typically used for therapy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com