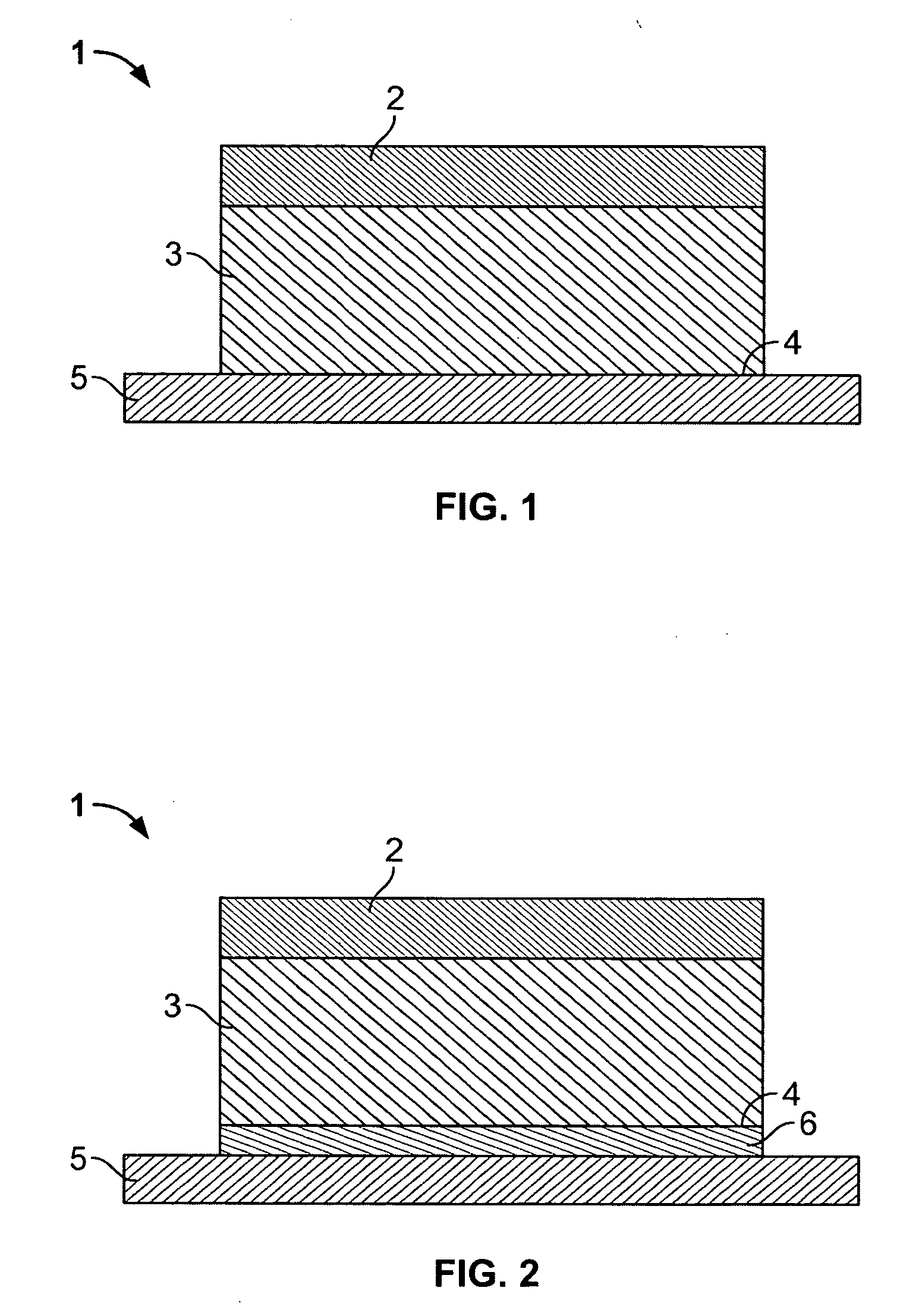
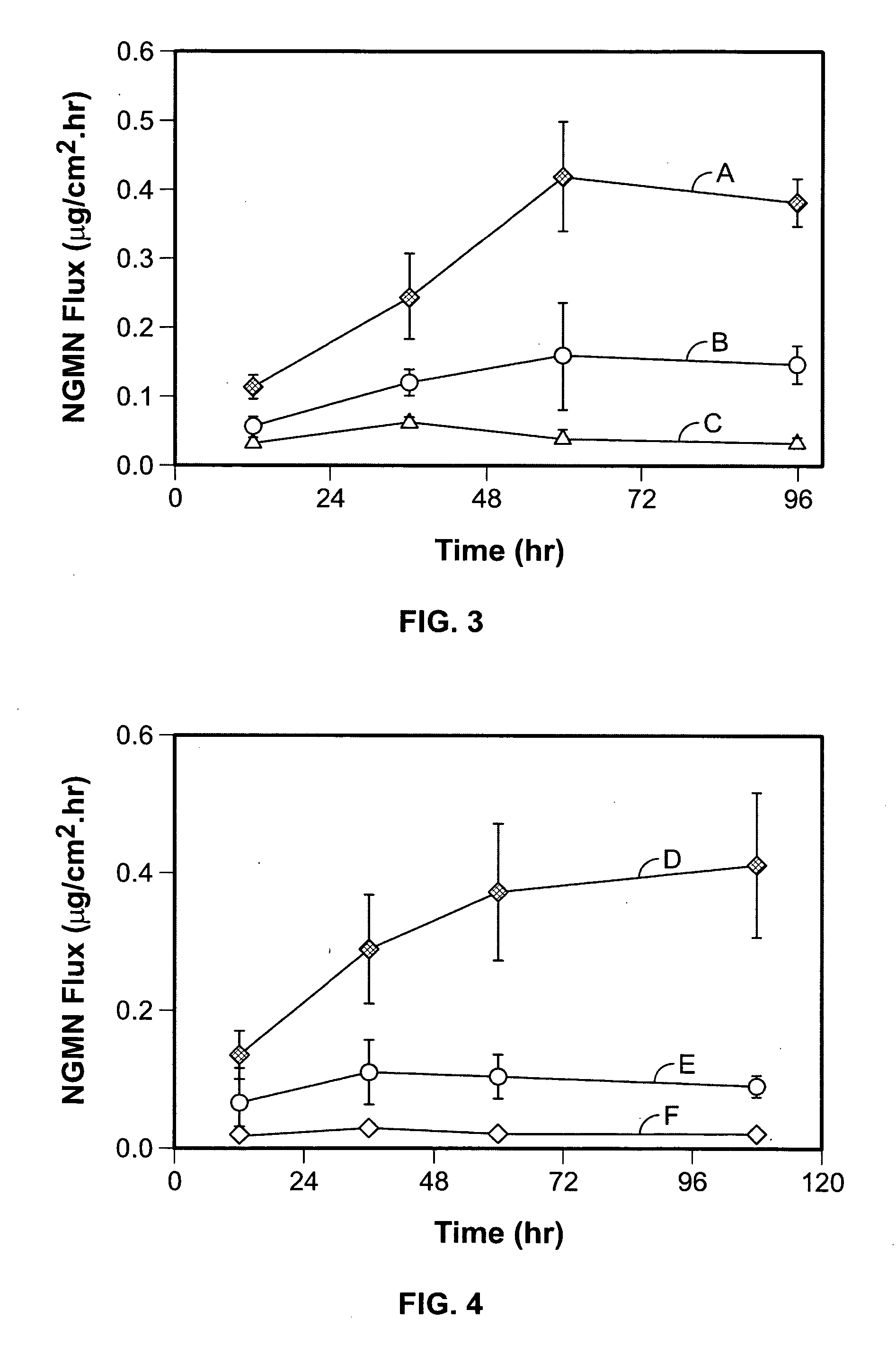
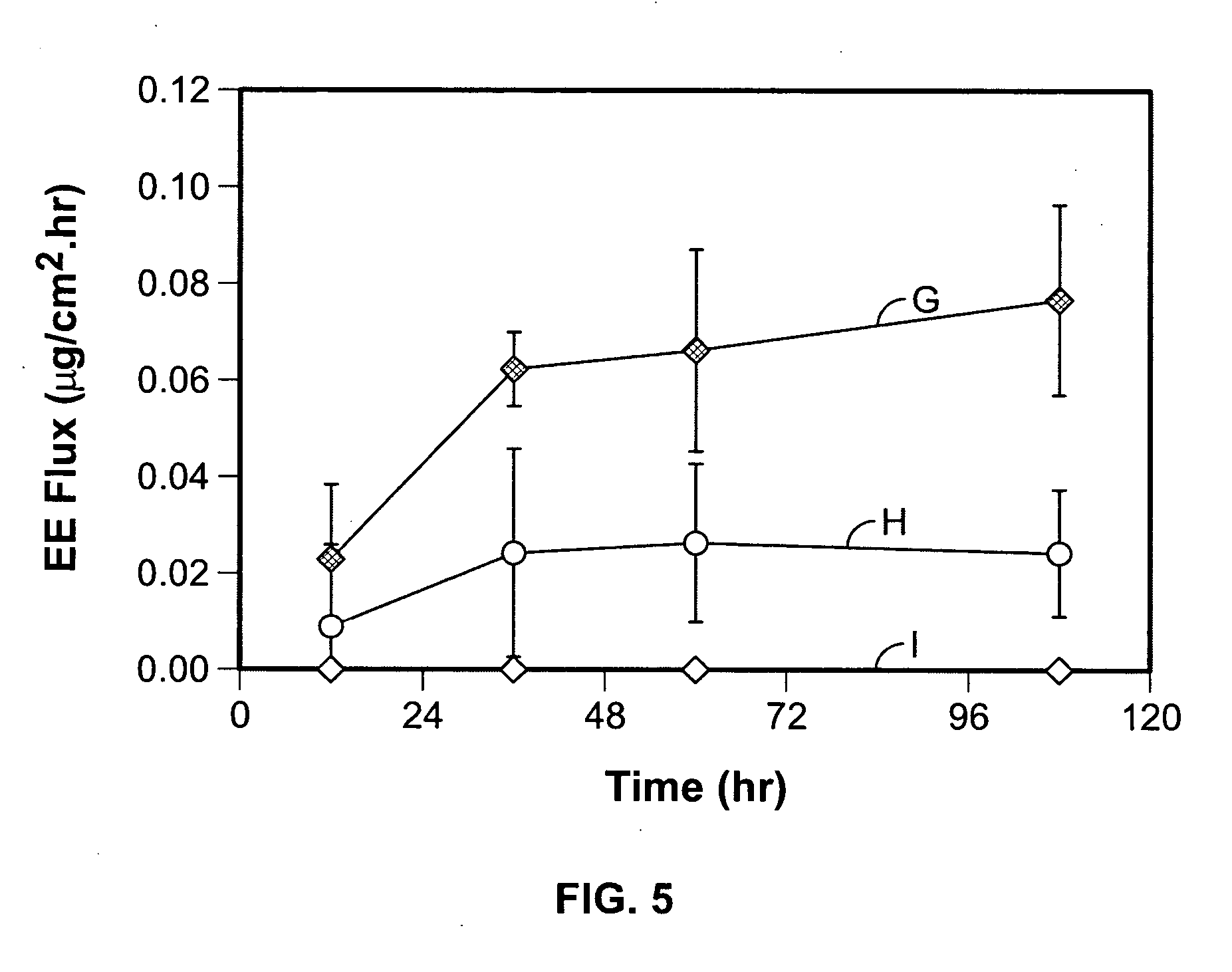
Transdermal norelgestromin delivery system
a norelgestromin and delivery system technology, applied in the field of medical patches, can solve the problems of high affinity for lint and dirt to adhere to the edge of the patch, severe cold flow, etc., and achieve the effects of low cost, high flux rate, and high loading rate of permeation enhancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0081] A monomer mix containing butyl acrylate, 2-hydroxyethyl acrylate, t-octyl acrylamide, acrylic acid, ethyl acetate (solvent), and 2,2′-azobisisobutyronitrile (AIBN) (polymerization initiator) was prepared. A fraction was charged to an appropriate vessel and heated to reflux with stirring. The remainder was added to the vessel over time. The ratios of the monomers and initiator added totally, i.e., butyl acrylate: 2-hydroxyethyl acrylate: t-octyl acrylamide: acrylic acid: AIBN were 59: 25.5: 9.5: 6: 2. The material was then held at reflux for a suitable period of time. At the end of the hold period, the contents were cooled to room temperature and the solution polymer discharged. The dry film made from this polyacrylate formulation had storage modulus of around 9×105 dyn / cm2, creep compliance of around 7×10−5 cm2 / dyn, and glass transition temperature of −8° C., and consequently was too stiff to provide adequate adhesive properties alone. This formed a proadhesive.
example 2
[0082] A monomer mix containing butyl acrylate, 2-hydroxypropyl acrylate, t-octyl acrylamide, acrylic acid, ethyl acetate (solvent), and 2,2′-azobisisobutyronitrile (AIBN) (polymerization initiator) was prepared. A fraction was charged to an appropriate vessel and heated to reflux with stirring. The remainder was added to the vessel over time. The material was held at reflux for a suitable period of time. The ratios of the monomers and initiator added totally, i.e., butyl acrylate: 2-hydroxypropyl acrylate: t-octyl acrylamide: acrylic acid: AIBN were 59: 25.5: 9.5: 6: 2. At the end of the hold period, the contents were cooled to room temperature and the solution polymer discharged. The dry film made from this polyacrylate formulation had storage modulus of around 8×105 dyn / cm2, creep compliance of around 4×10−5 cm2 / dyn, and glass transition temperature of −8° C., and consequently was too stiff to provide adequate adhesive properties alone. This formed a proadhesive.
example 3
[0083] A monomer mix containing vinyl acetate, 2-hydroxyethyl acrylate, 2-ethylhexyl acrylate, ethyl acetate (solvent), and 2,2′-azobisisobutyronitrile (AIBN) (polymerization initiator) was prepared. A fraction was charged to an appropriate vessel and heated to reflux with stirring. The remainder was added to the vessel over time. The material was held at reflux for a suitable period of time. The ratios of the monomers and initiator added totally, i.e., vinyl acetate: 2-hydroxyethyl acrylate: 2-ethylhexyl acrylate: AIBN were 50: 10: 40: 1.2. At the end of the hold period, the contents were cooled to room temperature and the solution polymer discharged. The dry film made from this polyacrylate formulation had storage modulus of around 2×106 dyn / cm2, creep compliance of around 4×10−6 cm2 / dyn, and glass transition temperature of −14° C., and consequently was too stiff to provide adequate adhesive properties alone. This formed a proadhesive.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com