Crf receptor antagonists, their preparations, their pharmaceutical composition, and their uses
a technology of receptor antagonists and receptors, applied in the field of receptor antagonists, can solve the problems of short half-life and limit the usefulness of receptor antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
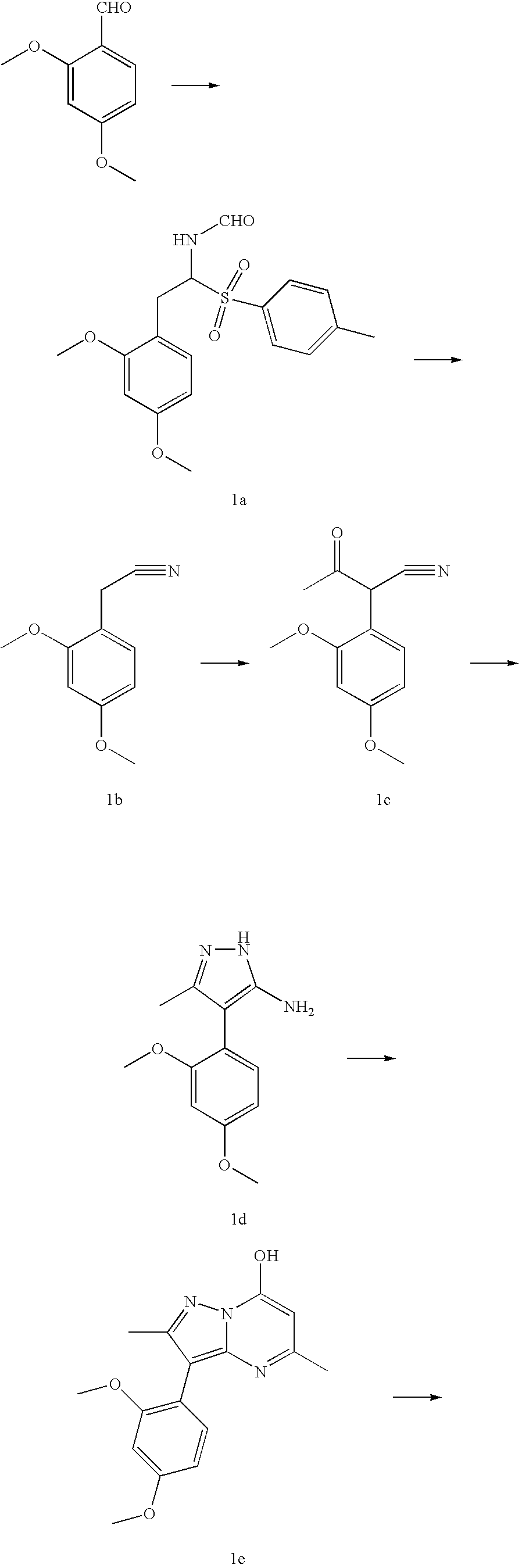
[0032]
Step 1A:
[0033] A suspension of potassium t-butyloxide (7.3 g, 65 mmol, 1.4 eq) in 1,2-dimethoxyethane (DME, 40 mL) was chilled to −50° C. under nitrogen. Tosylmethyl isocyanide (9.1 g, 46.5 mmol, 1 eq) in 40 mL DME was added dropwise while the temperature was kept below −5020 C. 2,4-Dimethoxybenzaldehyde (7.7 g, 46.5 mmol, 1 eq) was added dropwise and the reaction mixture was stirred for 30 minutes to give compound 1a. MeOH (100 mL) was added and the mixture was refluxed for 30 minutes. Most of the DME and MeOH were removed, the residue was resuspended in water (100 mL) and ethyl acetate (100 mL). Following neutralization with acetic acid, the organic layer was washed with brine and dried under sodium sulfate, concentrated and purified by silica gel column using 25% ethyl acetate in hexane to yield 5.5 g of 1b as a white solid.
Step 1B:
[0034] To 2,4-dimethoxyphenylacetonitrile 1b (36.0 g, 204 mmol, 1 equiv.) in dry THF (300 mL) was added 12 g (510 mmol, 2.5 equiv) of 60% N...
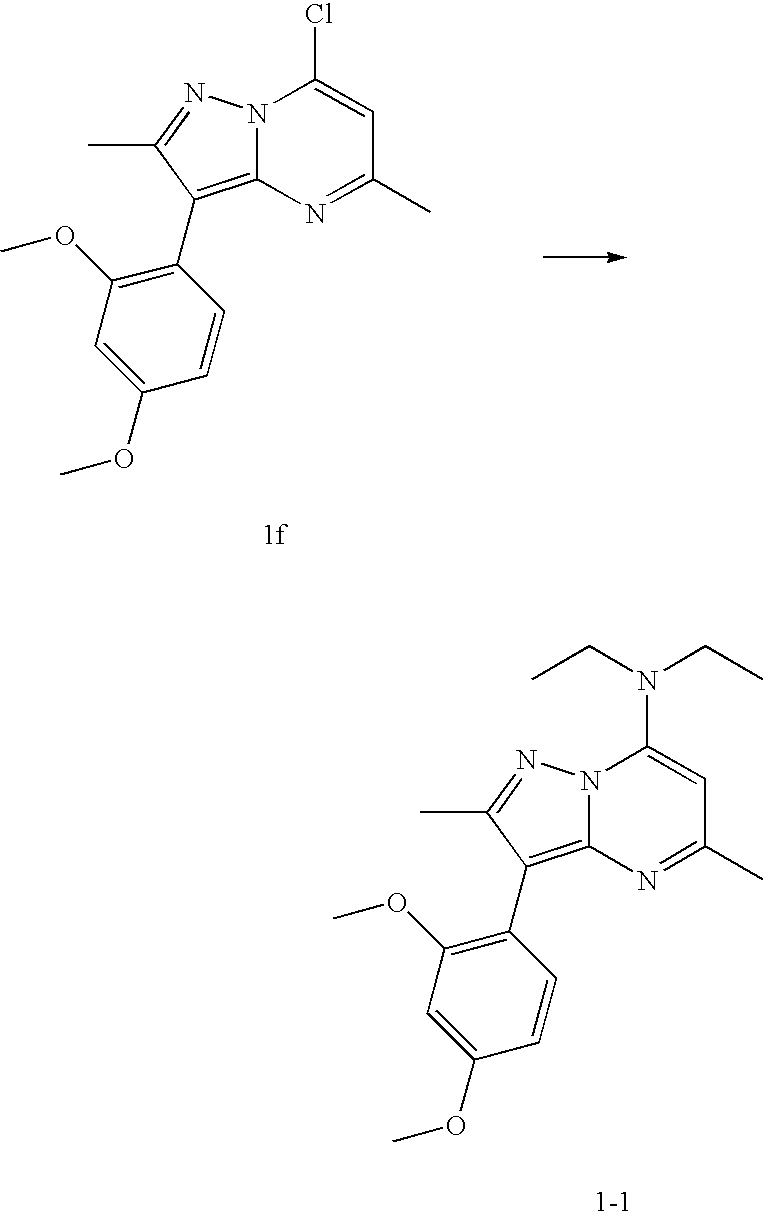
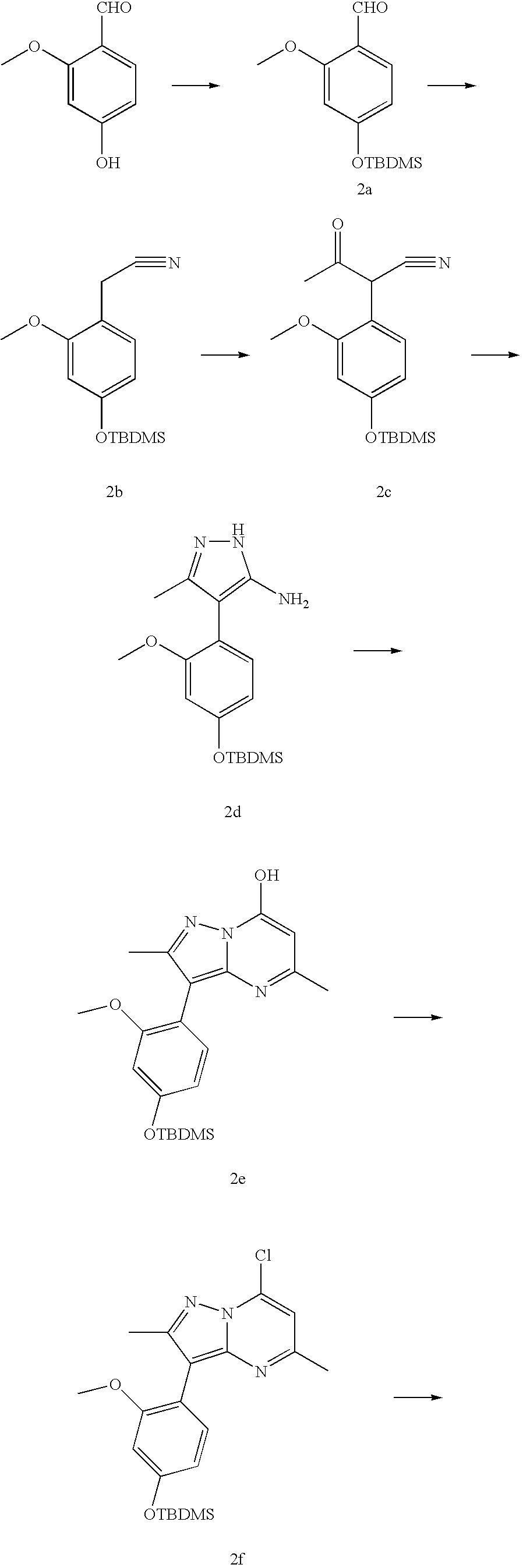
example 2
[0042]
Step 2A:
[0043] 2-Hydroxy-4-methoxybenzaldehyde is protected by reaction with tert-butyldimethylsilyl chloride (TBDMSCl) and imidazole in DMF to give the tert-butyldimethylsilyl ether 2a. Using 2a and following the procedure as outlined in steps 1A to 1E, compound 2f is realized. 2f and diethylamine following the procedure of Step 1F followed by deprotection of the tert-butyldimethylsilyl group with tetrabutylammonium fluoride or acid under standard conditions gives 2g. Alkylation of the hydroxy group of 2g with 11C methyl iodide and sodium hydride in solvent such as DMF or acetonitrile gives 2-1.
example 3
[0044]
Step 3A:
[0045] Compound 1f and ethylaminoethanol following the procedure of Step 1F gives 3a.
Step 3B:
[0046] Compound 3a is converted to the sulfonate ester such as mesylate, tosylate or triflate followed by reaction with 18F ion to give 3-1 (L. Martarello et al., Nuclear Medicine and Biology 28 (2001) 187-195). Compound 3a and methanesulfonyl chloride in pyridine yields a mesylate which undergoes a nucleophilic substitution with K18F to give 3-1 which is purified by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pharmaceutically acceptable | aaaaa | aaaaa |
| Disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com