Therapeutic Agent for Polycystic Ovary Syndrome (Pcos)
a polycystic ovary syndrome and therapeutic agent technology, applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of imbalance between fsh, hormone, lh (luteinizing hormone) in gonadotropics, and is not properly maintained, so as to achieve excellent induction ovulation effect, safe availability, and few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
Preparation of the extract of mushrooms (Grifola frondosa)
[0056] 5 L of 95% aqueous ethanol was added to 1000 g of dried Grifola frolndosa) fruiting body powder, stirred for 2 to 3 hours at room temperature, and then filtered to remove the ethanol soluble components.
[0057] Then, 5 L of deionized water was added to the obtained residue, and then extracted for 2 hours while stirring at 100 to 120° C. This hot-water-extracted liquid was concentrated to half of the original value, followed by adding ethanol until the final ethanol concentration became 50 to 75% by value. This was allowed to stand in a low-temperature chamber at 4 to 10° C. for 8 to 12 hours, and solid substances such as precipitates or floating matter, which were insoluble components, were removed by centrifugation to obtain a supernatant.
[0058] From this supernatant, a fraction having a molecular weight of 14,000 Dalton or more was collected by dialysis, and thus an extract of Grifola frondosa was obtained.
[0059] I...
preparation example 2
Preparation Method of a Tablet
[0072] A tablet having the following components was prepared using the extract of Grifola frondosa which was obtained in Preparation Example 1.
TabletPer tabletActive ingredients:Extract of Grifola frondosa (powdered)36 mgDried Grifola frondosa powder250 mg Excipients:Fine crystalline cellulose264 mg Calcium monohydrogen phosphate20 mgSucrose fatty acid ester20 mgFine silica dioxide10 mgCoating material:Methylcellulose1 to 1.5% by mass of the tablet
[0073] The dried Grifola frondosa powder means a powder obtained by merely drying and pulverizing Grifola frondosa without extracting with ethanol. The above-mentioned extract of Grifola frondosa (powdered) and the dried Grifola frondosa powder were mixed with the finely pulverized excipients and granulated, dried, pulverized, and tableted to make tablets having a proper form and size, followed by coating the tablets with the coating material.
[0074] Results of physical analysis and microbial test on the ob...
preparation examples 3 to 6
Preparation and Analysis of Extracts From Lentinus edodes, Agaricus blazei Murill, Ganoderma lucidum, and Pleurotus ostreatus
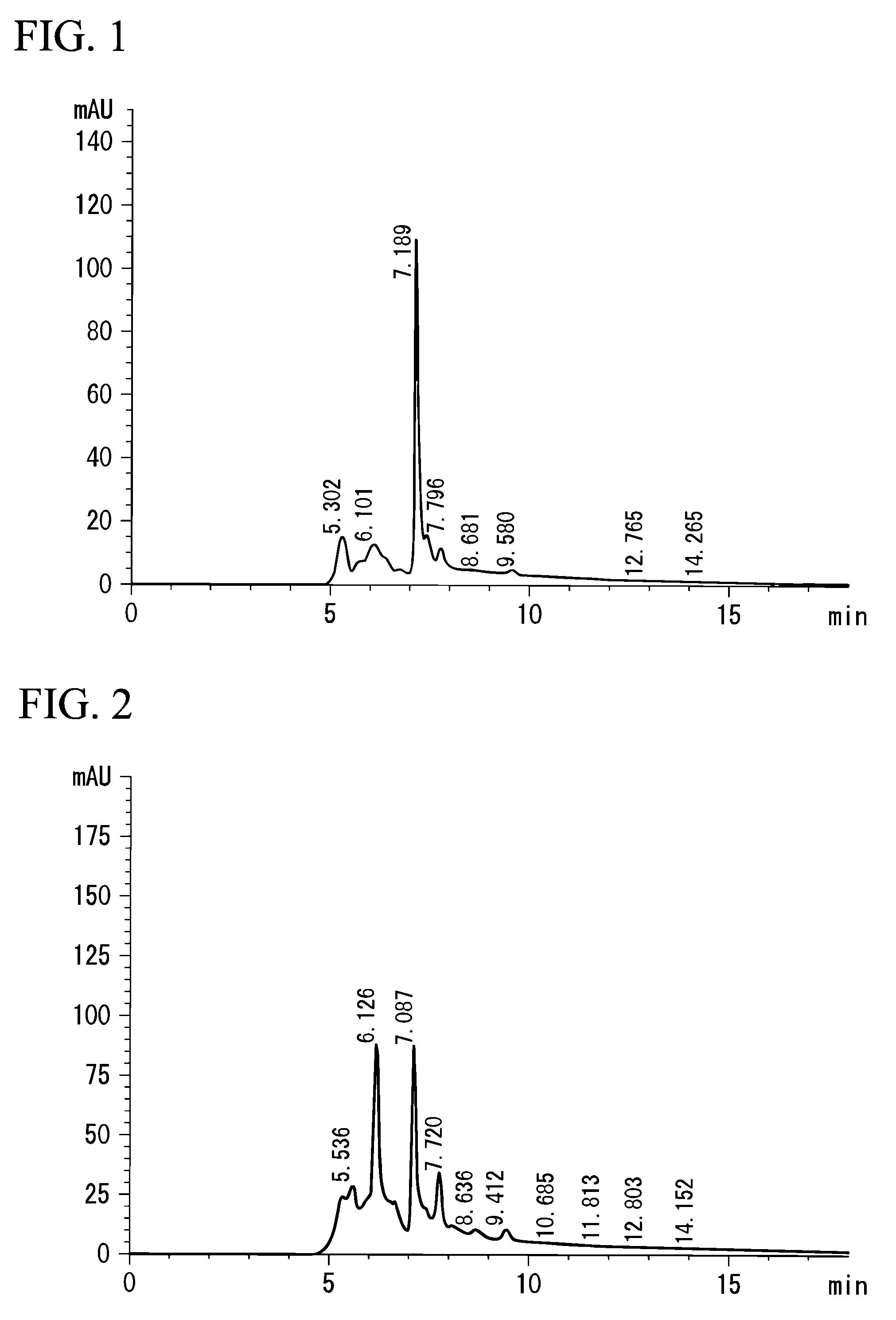
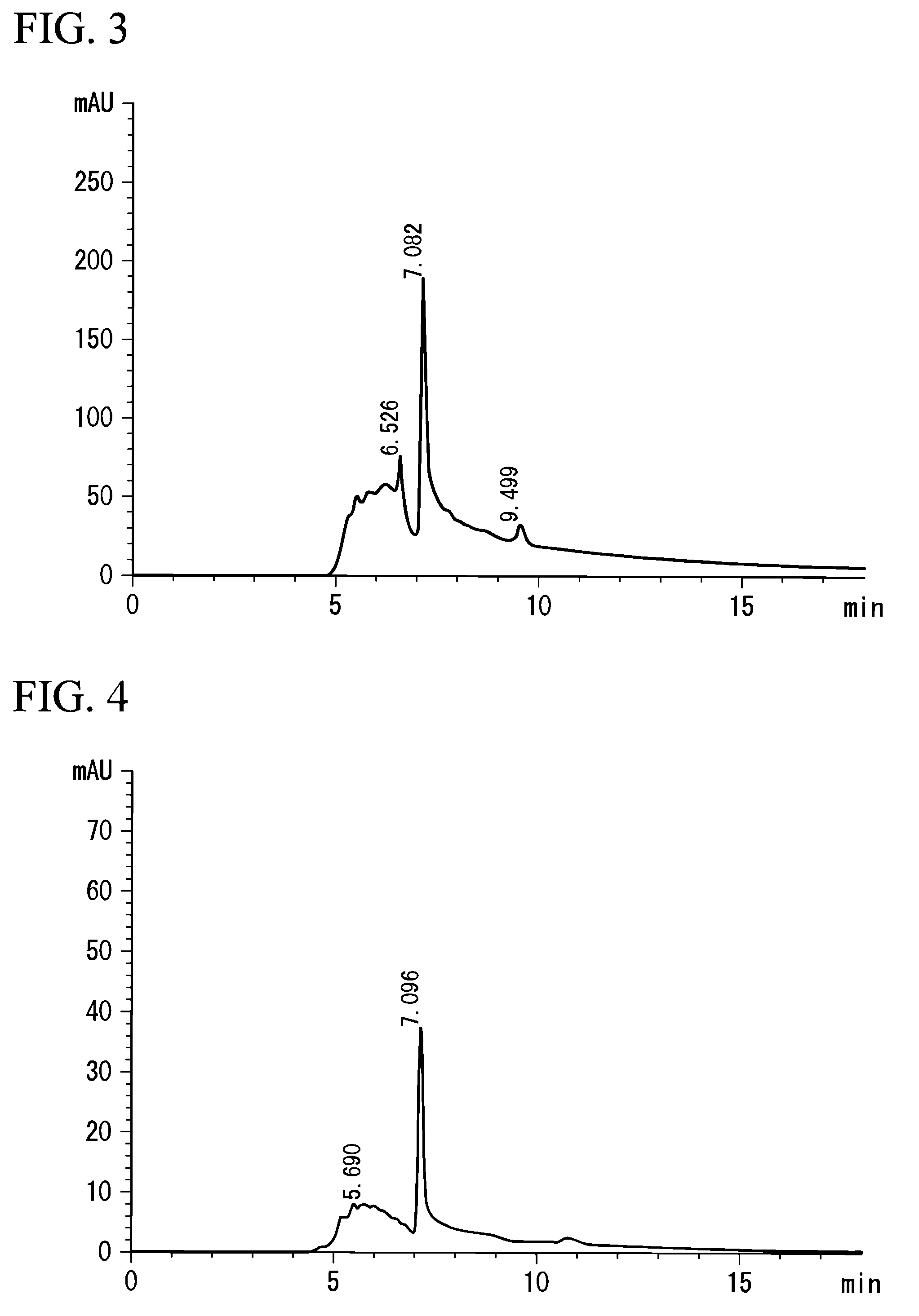
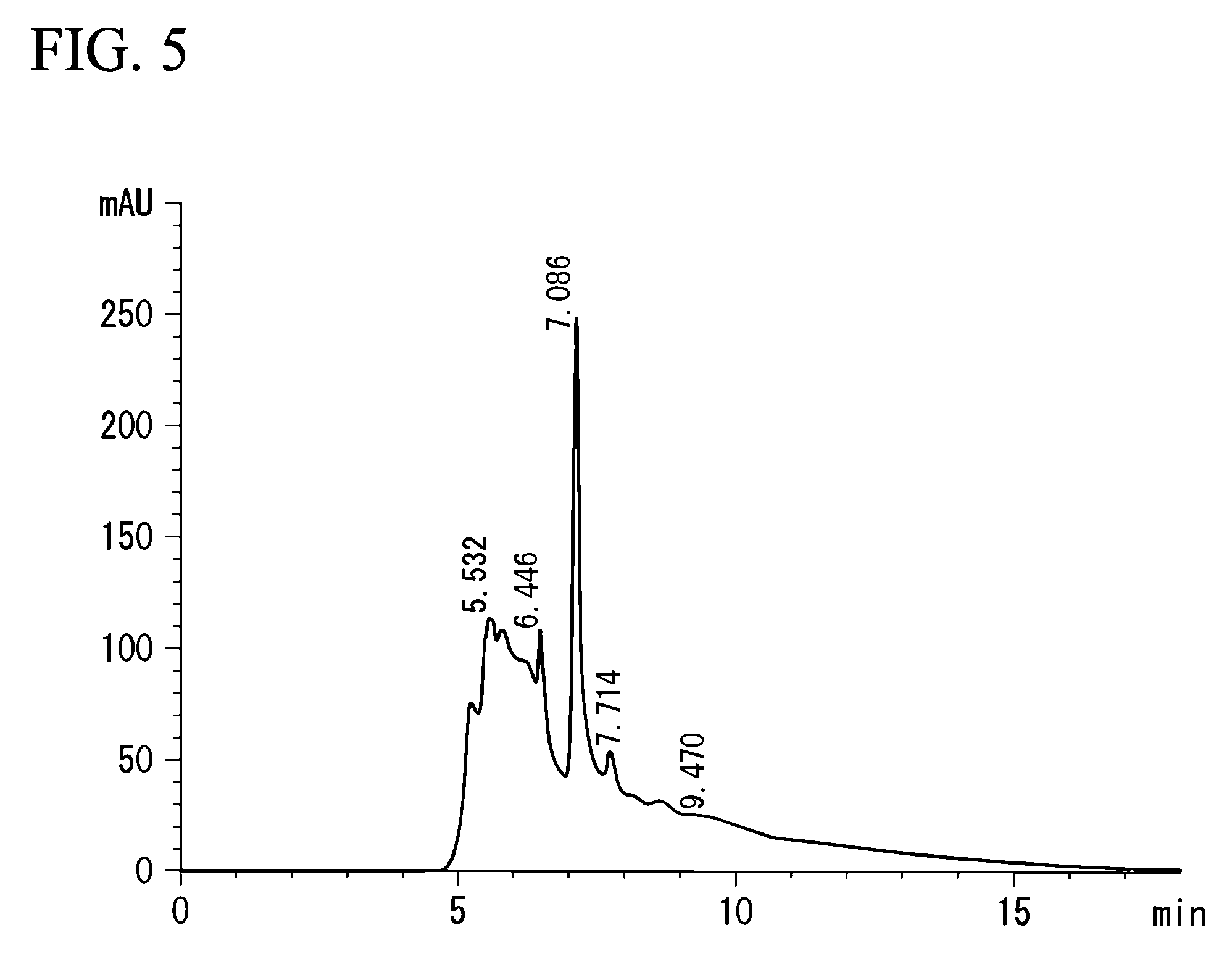
[0075]Lentinus edodes (Preparation Example 3), Agaricus blazei Murill (Preparation Example 4), Ganoderma lucidum (Preparation Example 5), and Pleurotus ostreatus (Preparation Example 6) were used instead of Grifola frondosa, for extraction in the same manner as that in Preparation Example 1. The measurements by HPLC were carried out in the same manner as in the above-mentioned analysis example. FIG. 2 shows a chart drawing of a result of the analysis by HPLC on the extract of Lentinus edodes extract, FIG. 3 shows that on Agaricus blazei Murill, FIG. 4 that on Ganoderma lucidum, and FIG. 5 shows that on Pleurotus ostreatus. In FIGS. 2 to 5, each peak was recognized at approximately the same position of 7.189, which is the peak position of the extract of Grifola frondosa in Preparation Example 1, that is, 7.087 (FIG. 2), 7.082 (FIG. 3), 7.096 (FIG. 4), or 7.086...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com