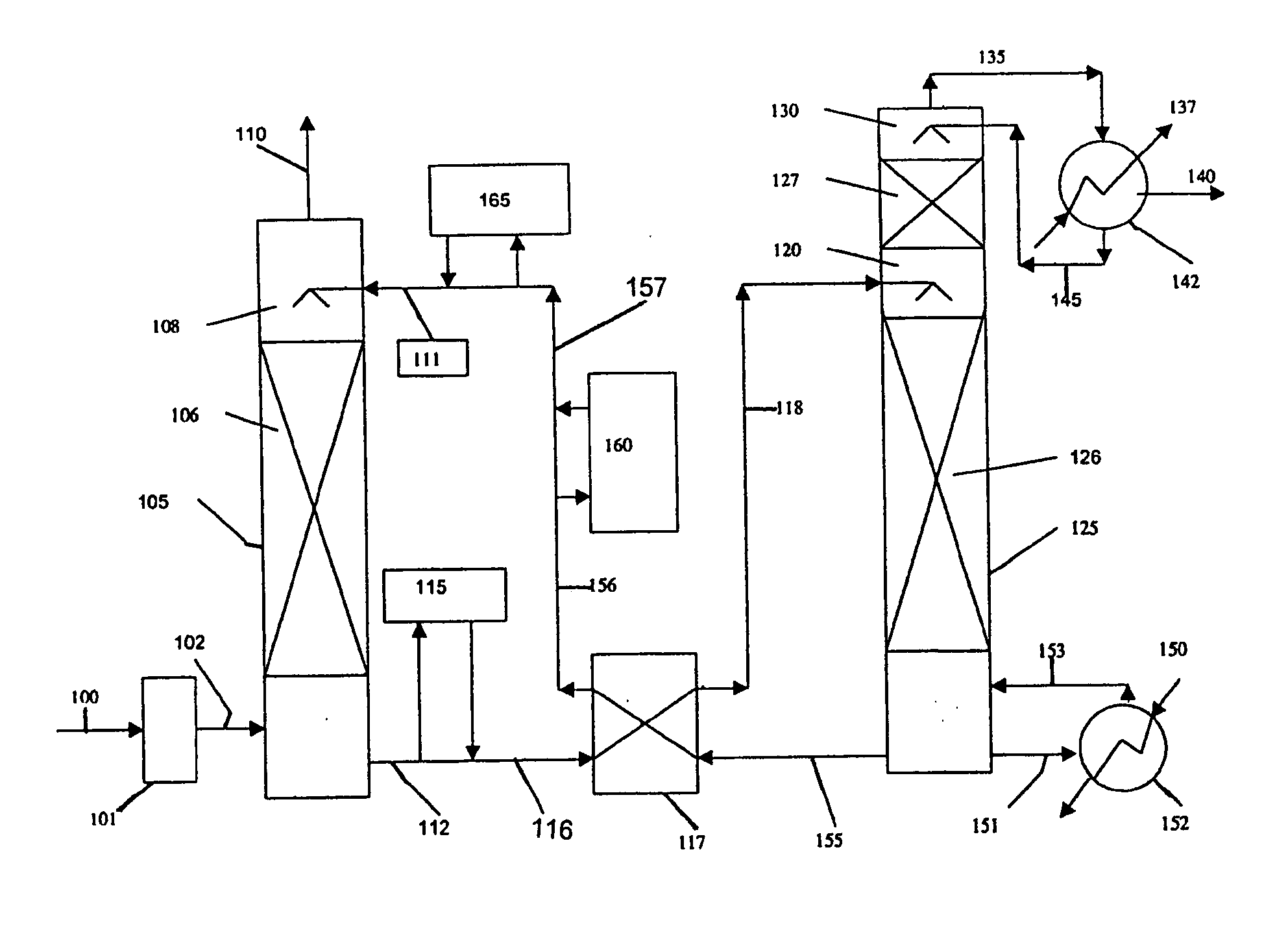
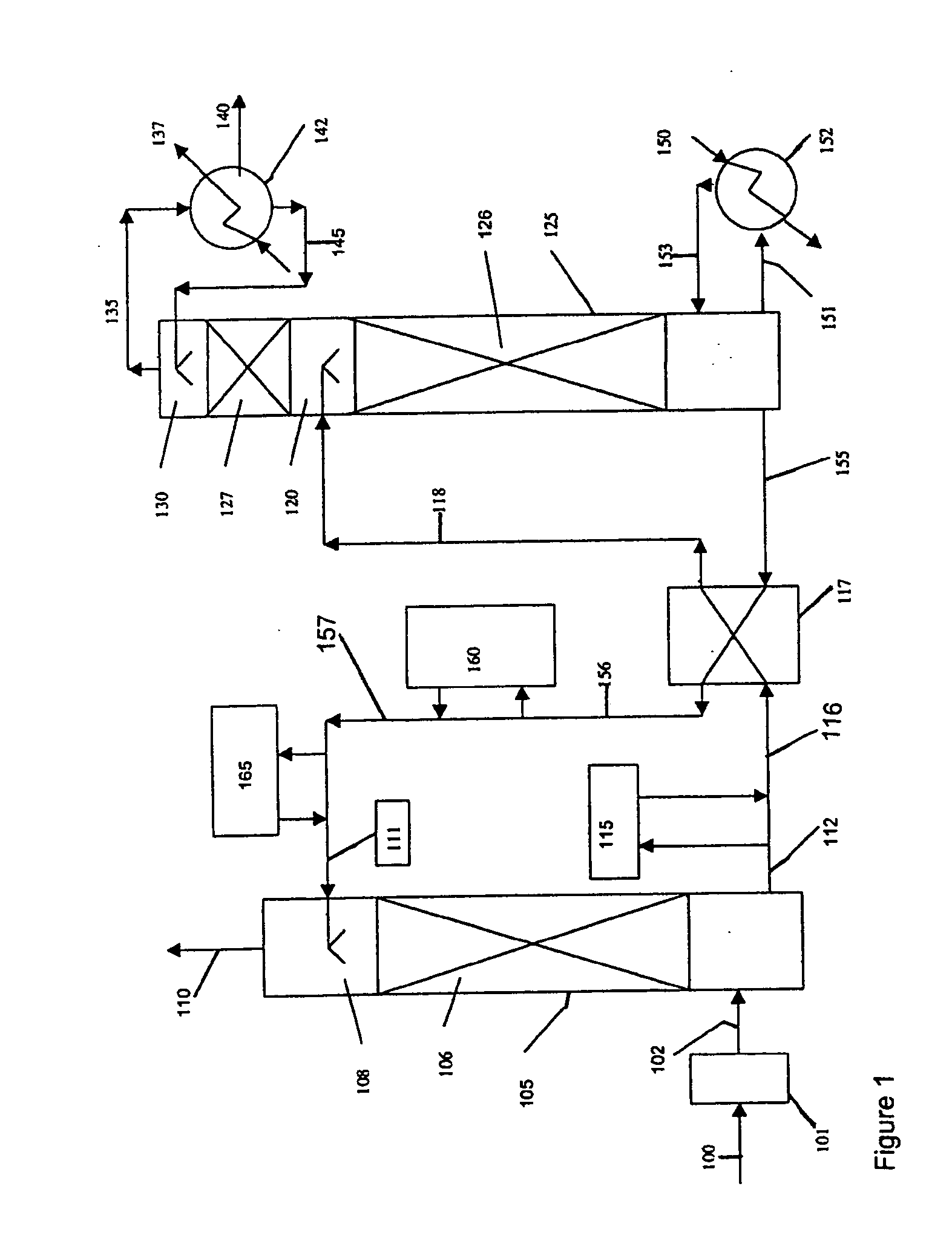
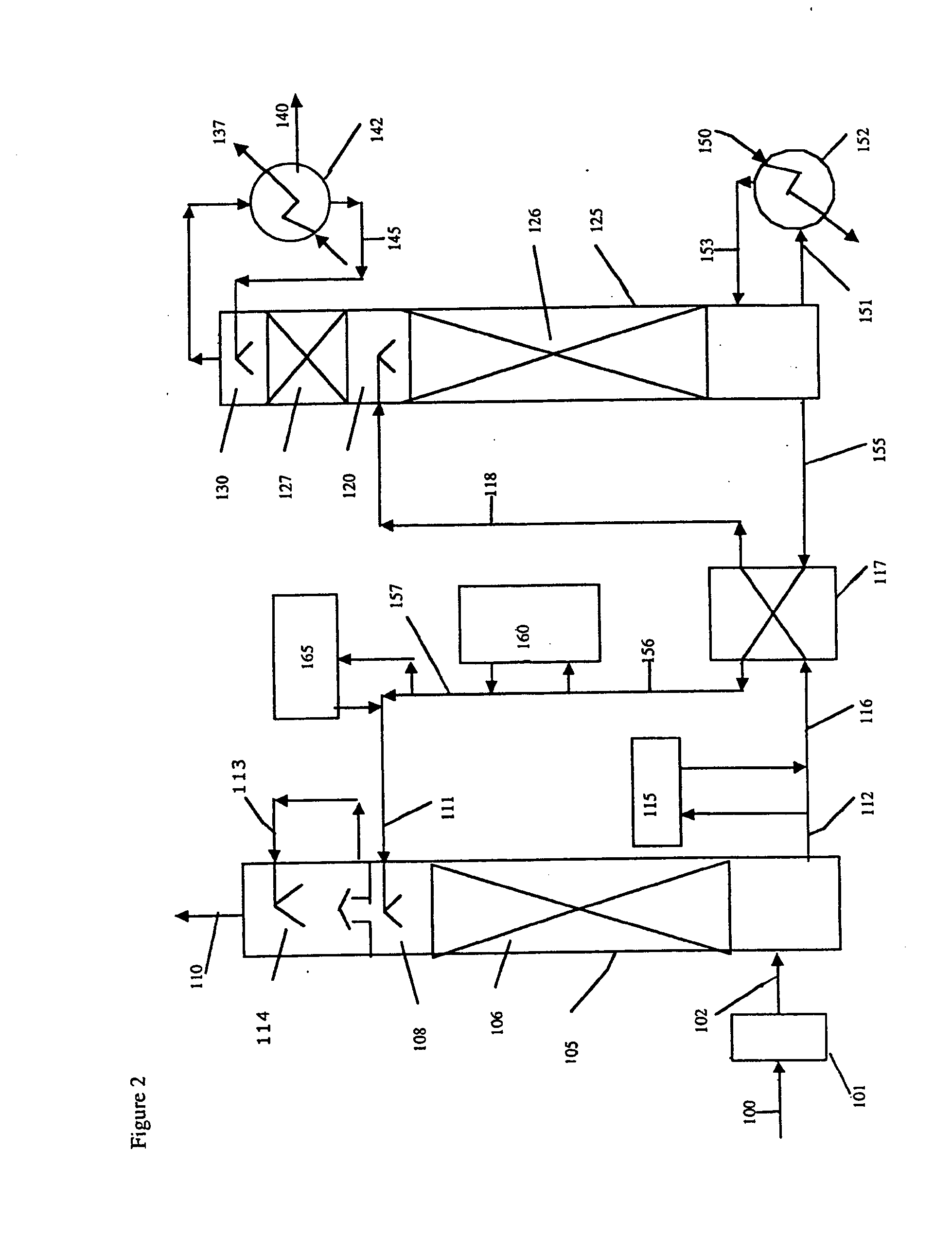
Method and apparatus for NOx and Hg removal
a technology of nox and hg, which is applied in the field of method and apparatus for nox and hg removal, can solve the problems of large equipment and the toxic and flammable nature of ammonia reductant, the inability to remove nox and hg, so as to reduce the rate of fe2+ oxidation, prevent buildup, and stabilize iron edta
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0079] The DeNOx (NOx removal) performance and the rate of Fe2+ oxidation in an uninhibited 0.1 M solution of Fe2+ EDTA was measured. The absorber has 12″ of packing, the absorber and regenerator sump oil baths were kept at 60° C. and 115° C. respectively. The absorbent flow rate was 10 ml / min and the gas flow was 2.3 l / min with a composition of 5% O2 and 580 ppmv NO, 13 ppmv NO2 with the balance nitrogen. The treated gas contained undetectable NO2. The ferrous iron oxidized to Fe3+ in about 4 hours, with the % DeNOx decreasing accordingly. Addition of sodium dithionite (Na2S2O4) reducing agent increased the concentration of Fe2+ and % DeNOx. The absorbent pH dropped quickly from 5.7 to the 3-4 range. The results are given in the following table. The following is the definition of the heading of each column in the table.
Time, hoursNa2S2O4, gFe2+, %NOLean pH% DeNOx01005805.7—0.576483.9192151483.6792251953.49843381383.6764131453.657552533.15916168823.418672251093.758182251493.857491...
example 2
[0081] The experiment of Example 1 was repeated with 1.68 wt. % sulfite ion from sodium sulfite and 0.42 wt. % thiosulfate ion from sodium thiosulfate added to the absorbent. The NO and NO2 concentrations in the feed gas were 540 and 44 ppmv respectively. The feed gas flow rate was 2.3 l / min and contained 5 vol. % O2 and the absorbent rate was 10 ml / min. The treated gas contained undetectable NO2. The results are given in the table below.
Time, hoursNa2S2O5, gFe2+, %NOLean pH% DeNOx0100 5405.9—0.564255.8995%164225.9796%2N / A275.63*95%351305.6694%464375.6793%5364425.5792%651375.8193%6.5451405.8893%751435.64*92%7.5551405.7693%864335.7694%964305.8394%1051335.89*94%10.3351355.9494%
Note:
*Sulfuric acid added to maintain pH close to 5.9.
[0082] The addition of the sulfite and thiosulfate resulted in a higher and more stable % DeNOx. Sulfite was consumed by the oxidation process with the sulfite ion level decreasing to 0.68 wt. % in 4.5 hours. To maintain the presence of sulfite in the abs...
example 3
[0083] The test in Example 2 was continued with the same absorbent (i.e. the absorbent from the end of the run of Example 2), using sodium dithionite as a reducing agent reagent to maintain the Fe2+ concentration high enough to provide efficient DeNOx performance. The test data are given in the table below.
Time,hoursNa2S2O4, gFe2+, %NO% DeNOx04456611387487%1.51515590%2.51514093%3513195%41513993%5.51514193%6513494%71514991%81385291%9.51514293%10.51514193%11384692%11.51514492%
[0084] The consumption rate of dithionite reducing agent was 0.87 g / hr. The sulfite ion concentration dropped from 0.74 wt. % at time zero to 0.57 wt. 15% at 11.5 hours, while the thiosulfate concentration stayed constant at 0.34 wt. %, indicating that the dithionite was the major reagent being consumed. Sulfate ion increased from 1.66 to 1.92 wt. % over the 11.5 hour run. The absorbent pH remained essentially unchanged at pH 5.5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight % | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com