Curable polyvinyl benzyl compound and process for producing the same
a polyvinyl benzyl compound and polyvinyl benzyl technology, applied in the direction of synthetic resin layered products, woven fabrics, metal layered products, etc., can solve the problems of low crosslinking density, linear expansion coefficient, low chemical resistance,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
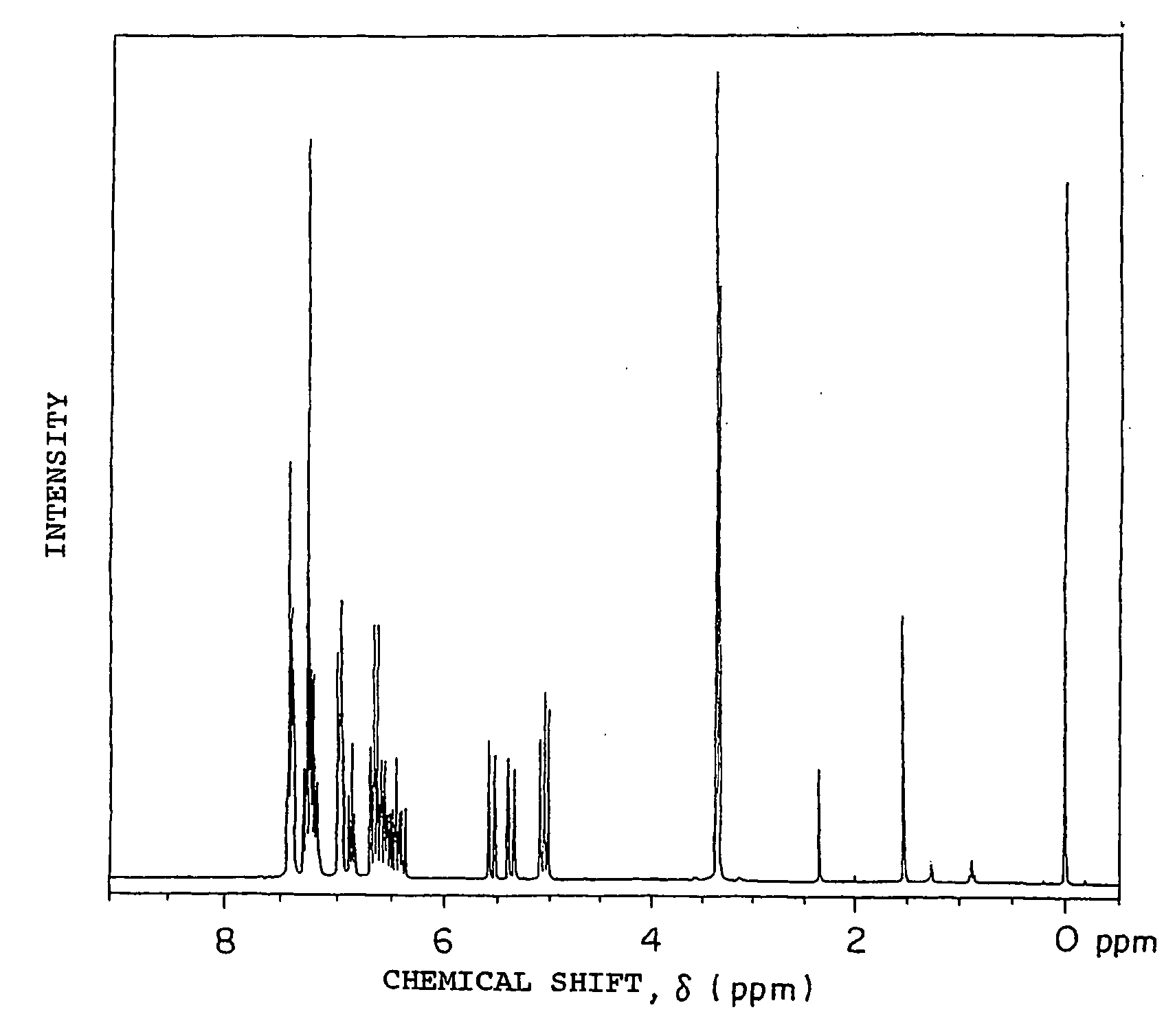
[0195] 49.8 g (0.3 mol) of fluorene, 200 g of methylisobutyl ketone, 2.91 g (9×10−3 mol) of tetra-n-butylammonium bromide, 0.73 g of hydroquinone and 96 g of a 50 wt % aqueous solution of NaOH (NaOH purity of 95%, 1.14 mol) were charged into a 1-liter four-necked flask equipped with a thermoregulator, stirrer, cooling condenser and dropping funnel and heated at 62° C. under agitation to prepare a uniform solution. 117 g of vinylbenzyl chloride CMS-AM (m- / p-isomers: 50 / 50 wt % mixture) available from Seimi Chemical Co., Ltd. (purity of 91%, 0.7 mol) was added dropwise to this dark blue green solution over 20 minutes and then a reaction was carried out at 60 to 61° C. for 7 hours. After 200 ml of toluene was added to the obtained green reaction product, the obtained solution was neutralized with 2N hydrochloric acid and washed with distilled water three times, toluene was removed under reduced pressure, and the obtained light yellow viscous solid was recrystallized from fresh toluene ...
example 2
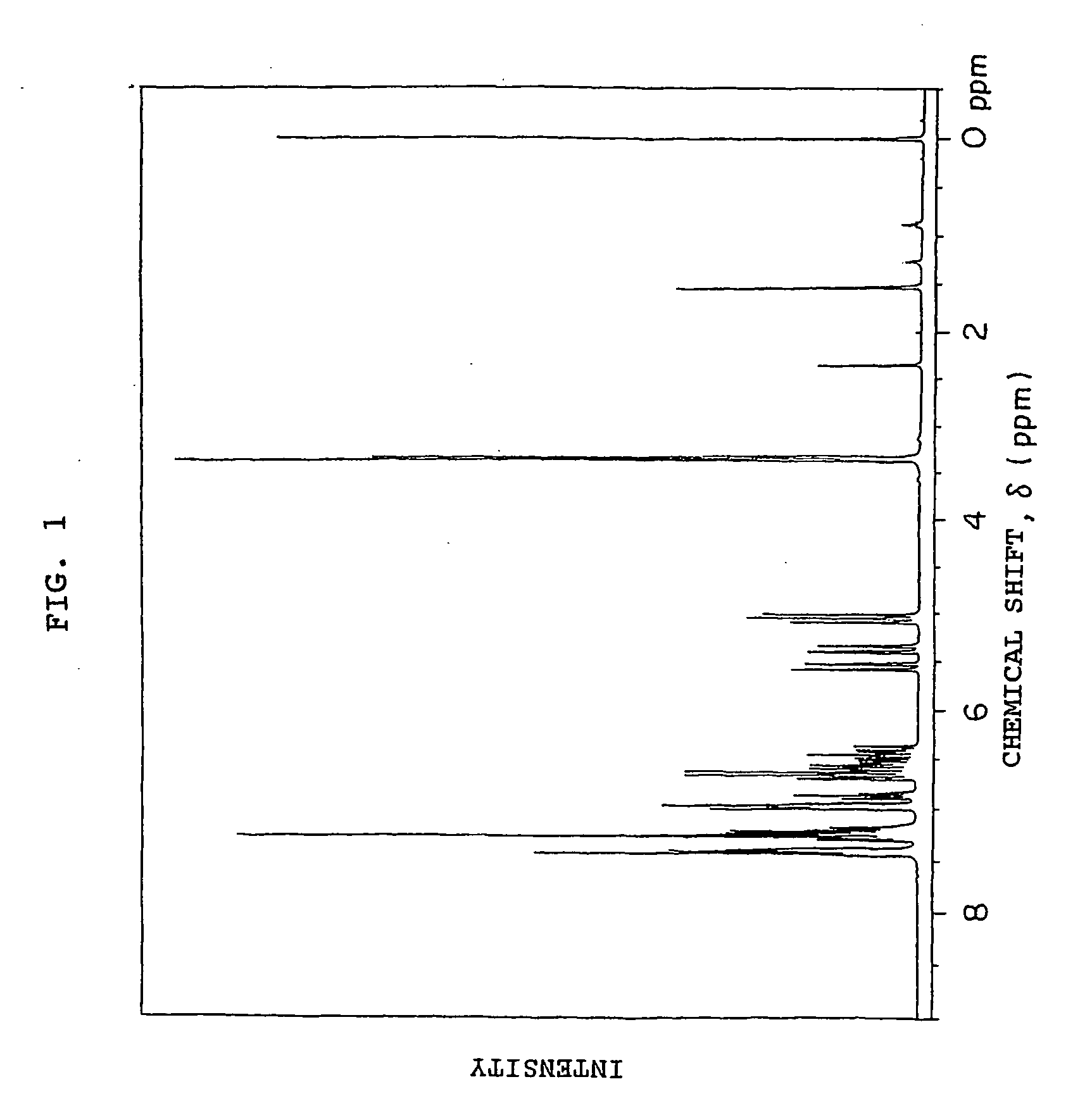
[0198] 49.8 g (0.3 mol) of fluorene, 220 g of toluene, 2.91 g (9×10−3 mol) of tetra-n-butylammonium bromide and 96 g of a 50 wt % aqueous solution of NaOH (purity of 95%, 1.14 mol) were added to the reactor used in Example 1 and heated at 65° C., and 21 g (0.12 mol) of p-xylylene dichloride was added, and reacted for 2.5 hours. After it was confirmed from the results of the 1H-NMR measurement of a small amount of the reaction product that p-xylylene dichloride was consumed, 54 g of CMS-AM (purity of 91%, 0.36 mol) was added dropwise to the reaction system and the reaction was continued at 65° C. for 6.5 hours. After the reaction solution was cooled to room temperature, 2N hydrochloric acid was added to neutralize the reaction mixture, and distilled water was added to the organic layer, which was then washed three times. After the solvent was distilled off under reduced pressure, the obtained solid was pulverized and filtered in methanol to collect solid matter through filtration, wh...
example 3
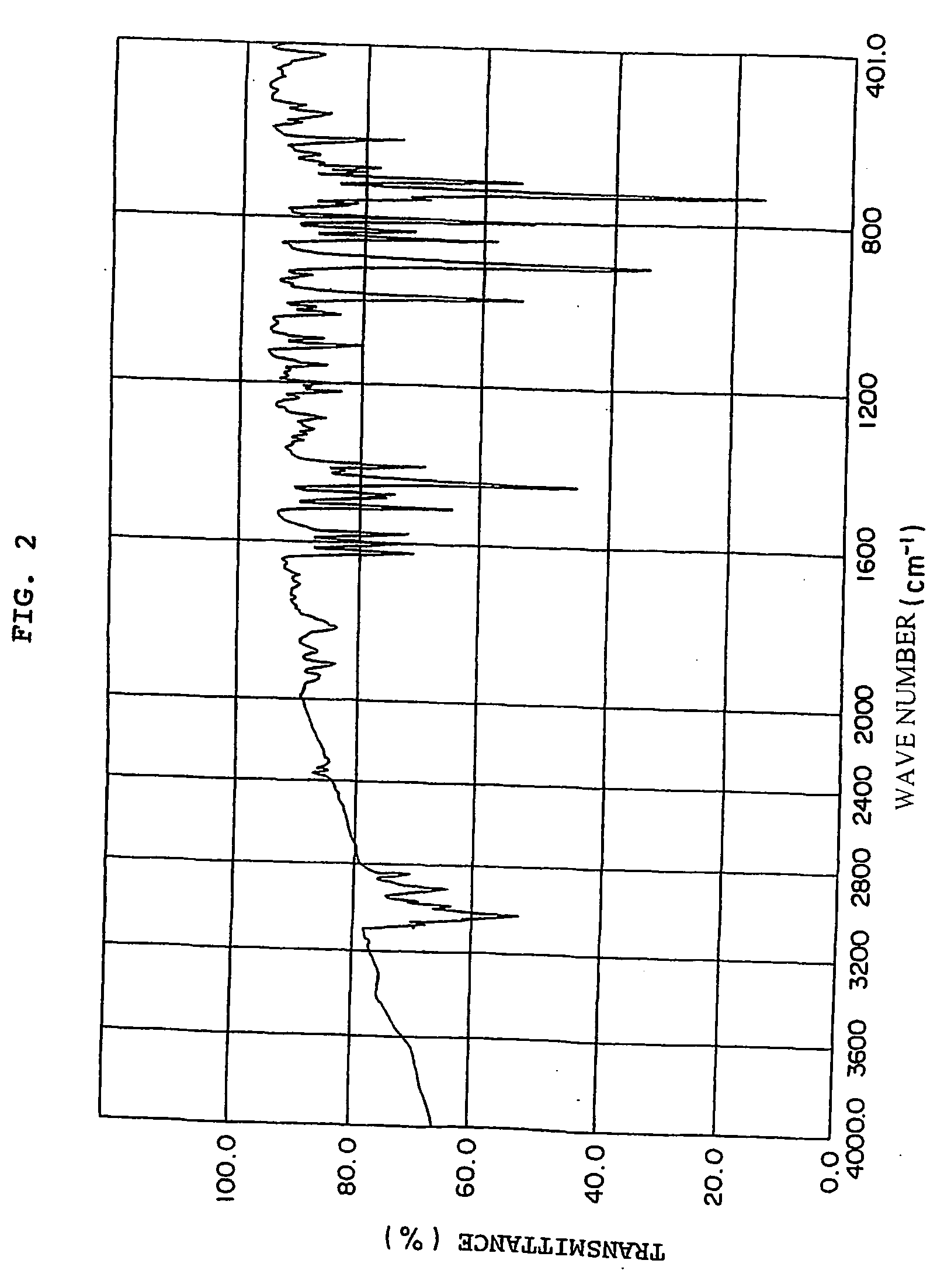
[0200] A solution containing 60 wt % of Compound 2 synthesized in Example 2 and 40 wt % of divinyl benzene (purity of 82%) was prepared, poured into the gap between glass plates and cured at 100° C. for 6 hours, at 160° C. for 4 hours and after-cured at 180° C. for 2 hours. Test specimens required for each measurement were prepared from the obtained resin plate. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com