Gastrointestinal Absorption Enhancer Mediated By Proton-Coupled Transporter and Its Preparing Method
a proton-coupled transporter and enhancer technology, which is applied in the direction of antibacterial agents, peptide/protein ingredients, drug compositions, etc., can solve the problems of insufficient pharmacological effects, cell damage, and inability to obtain substrate specificity in absorption, so as to improve the cellular absorption of pharmaceutical compositions and excellent gastrointestinal absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
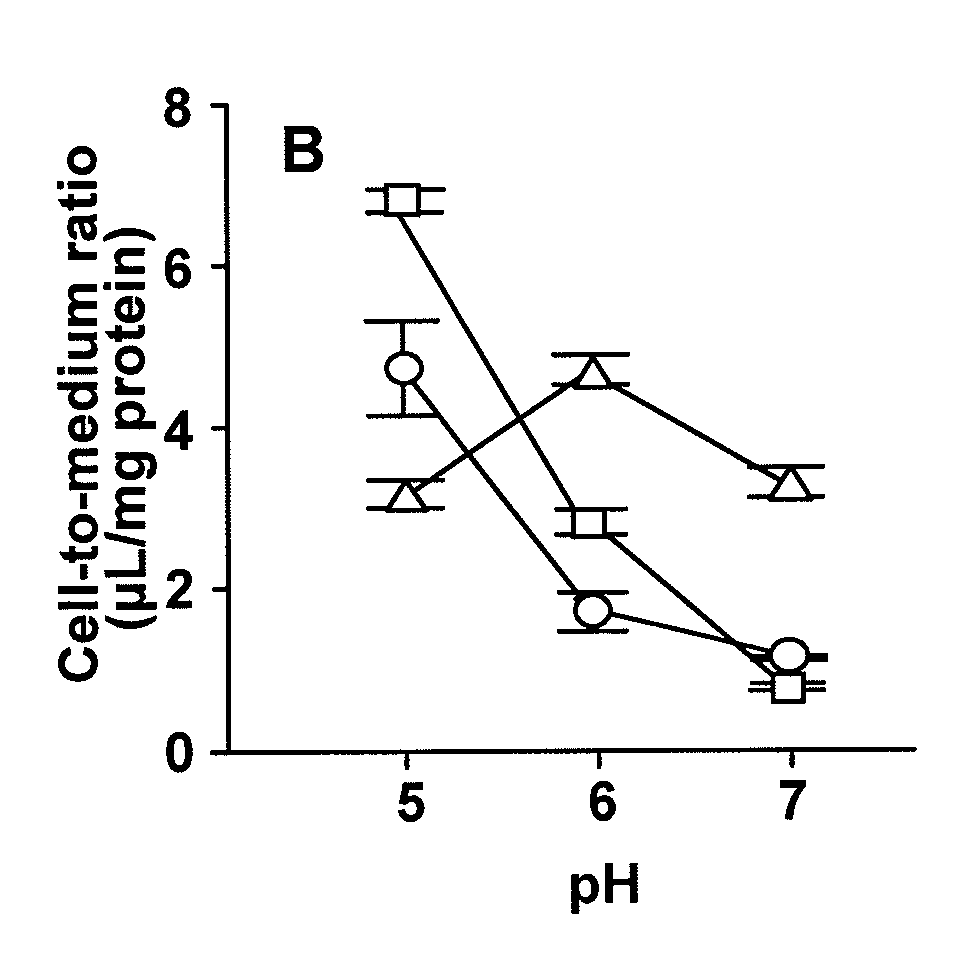
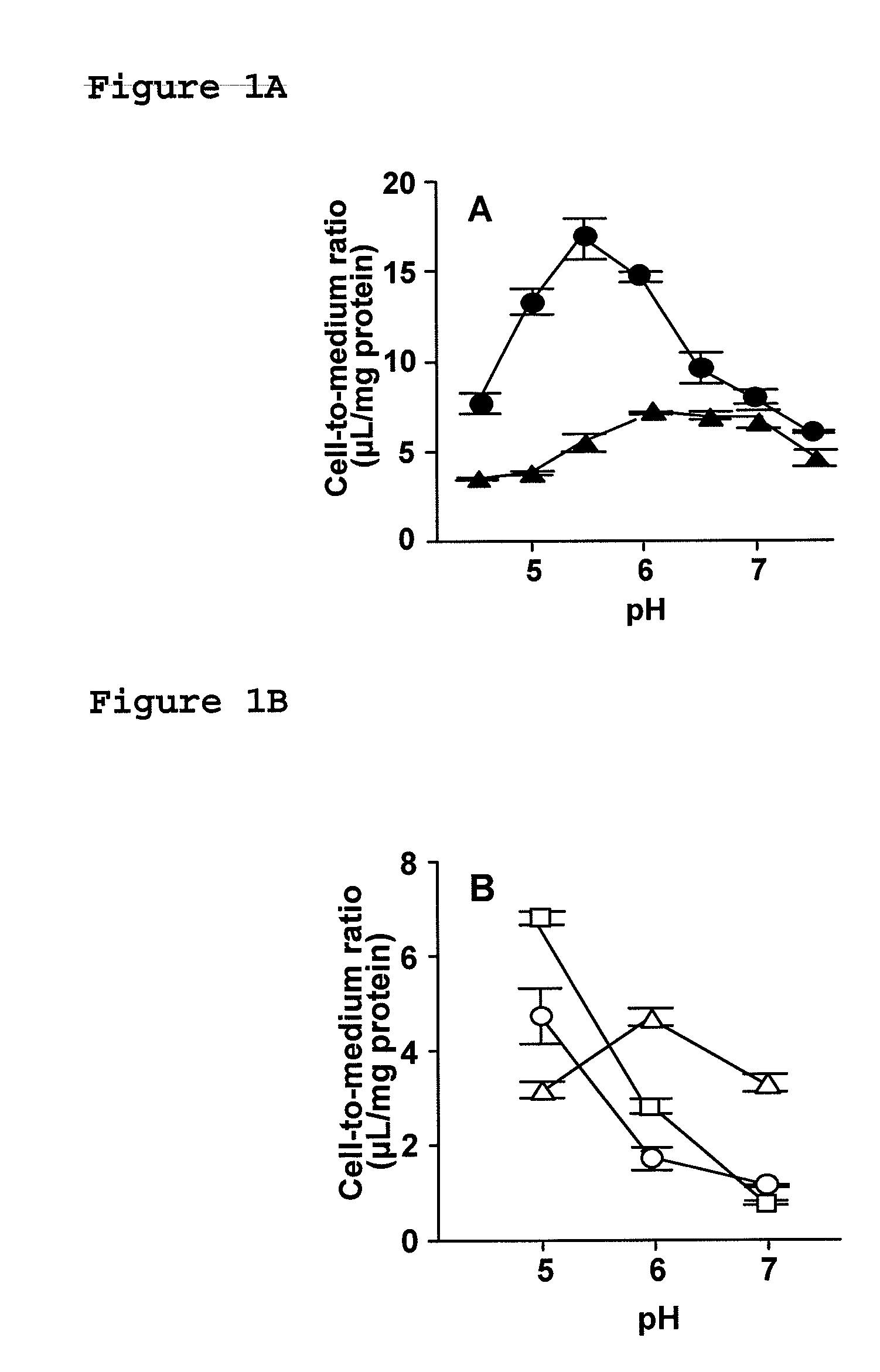
[0093]To investigate the effect of extracellular fluid on the cellular uptake of dipeptides and β-lactam antibiotics transported by PEPT1, the cellular uptake of dipeptides, i.e., [14C]glycylsarcosine ([14C]GlySar) and [3H]carnosine, and β-lactam antibiotics, i.e., cefadroxil (CDX), cefixime (CFIX), and FK089, was evaluated at pH 5.0 to 7.0 using gastrointestinal tract model cells (Caco-2 cells).
[0094]Cells cultured on 4-well plates were rinsed 3 times with 1 mL Hanks' balanced salt solution (HBSS: 0.952 mM CaCl2, 5.36 mM KCl, 0.441 mM KH2PO4, 0.812 mM MgSO4, 136.7 mM NaCl, 0.385 mM Na2HPO4, 25 mM D-glucose, 10 mM HEPES; pH 7.4; osmotic pressure 315 mOs / kg) heated to 37° C., and uptake was initiated by adding 250 μL HBSS containing medical fluid. Uptake was terminated at a predetermined period of time by washing the cells 3 times with 1 mL ice-cooled HBSS. After the completion of uptake, 0.25 mL 5 N NaOH was added, and the cells were agitated for 2 hours to solubilize, followed by n...
example 2
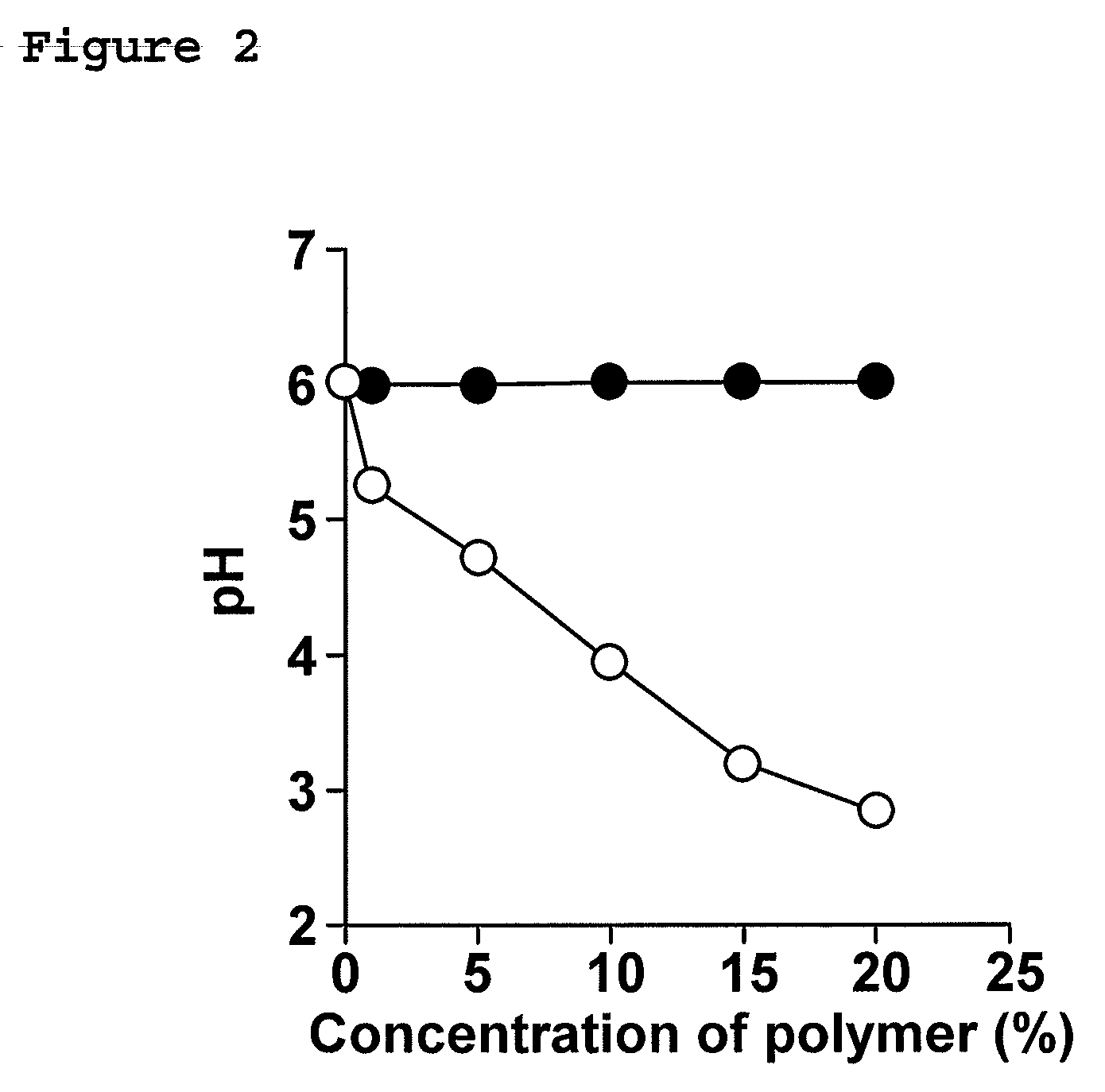
[0100]To investigate whether it is possible to control pH by adding pH-sensitive polymers, the effect of such a polymer on the pH of MES buffer was examined.
[0101]A methacrylic acid copolymer (Eudragit L100-55) was used as a pH-sensitive polymer, and an aminoalkyl / methacrylate copolymer (Eudragit RS PO) was used as a pH-insensitive polymer.
[0102]A polymer (methacrylic acid copolymer Eudragit L100-55 or, of a pH-insensitive polymer, aminoalkyl / methacrylate copolymer Eudragit RS PO) was added to MES buffer (pH 6.0). Subsequently, the pH of the buffer was measured by a pH meter.
[0103]The pH of the buffer decreased as Eudragit L100-55 was added (FIG. 2). In FIG. 2, plots () represent the pH of the MES buffer containing Eudragit RS PO, and plots (◯) represent the pH of the MES buffer containing Eudragit L100-55. Compared with the pH of the buffer having no polymer content, pH was decreased to about 3.0 when Eudragit L100-55 was added to a proportion of 20%. In contrast, the use of Eudra...
example 3
[0104]To investigate whether gastrointestinal absorption in rats of β-lactam antibiotics under physiological conditions can be improved by controlling the gastrointestinal pH, absorption of a zwitterionic compound (CDX) and an anionic compound (CFIX) in the presence and absence of a pH-sensitive polymer (Eudragit L100-55) was examined using the in situ closed loop method (FIG. 6 shows a diagram).
[0105]The oral composition of the invention was prepared by adding a β-lactam antibiotic (CDX or CFIX) to 10 mM MES buffer (pH 6.0) such that the buffer contained CDX in an amount of 1 mM or CFIX in an amount of 0.5 mM, and further adding Eudragit L100-55 to the buffer so that it contained in a proportion of 10 or 20 wt. % based on the amount of the entire oral composition. A β-lactam antibiotic solution containing no Eudragit L100-55 was prepared as a control.
[0106]These compositions were administered into the intestinal loops prepared at the caecum junction of SD male rats (junction betwee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| permeability | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com