Coated Microstructures and Methods of Manufacture Thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fabrication of Coated Microneedles
[0117]Metal microneedles were laser cut, electropolished, and then coated with various coating materials. Coated single microneedles and coated microneedle arrays were produced.
[0118]Forming the Microneedle Structures
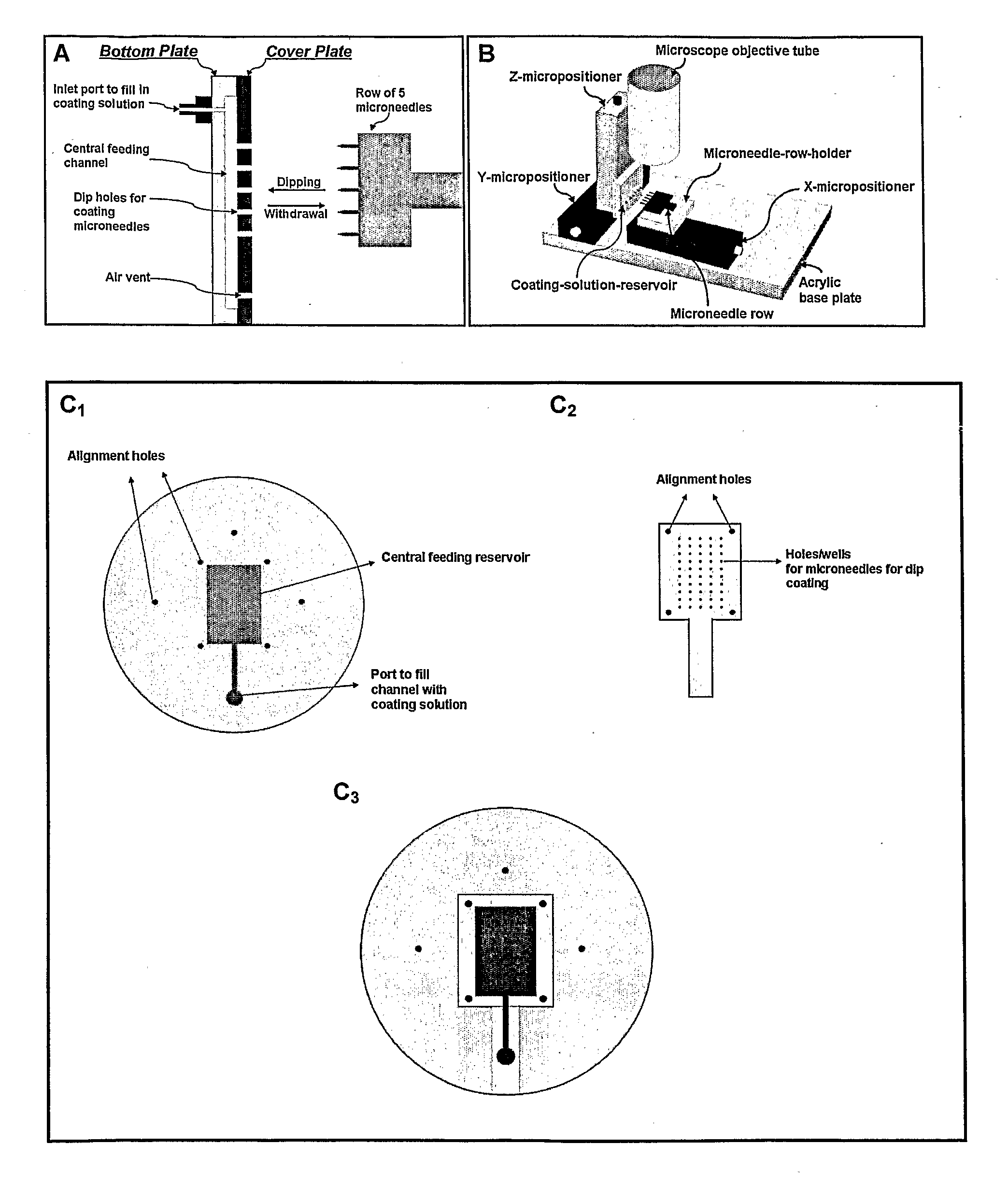
[0119]Solid microneedles were cut from stainless steel sheets (Trinity Brand Industries, SS 304, 75 μm thick; McMaster-Carr, Atlanta, Ga., USA) using an infrared laser (Resonetics Maestro, Nashua, N.H., USA), guided by CAD / CAM design, using techniques known in the art. Microneedles were prepared as single microneedles, individual rows of microneedles, or as two-dimensional arrays of microneedles.
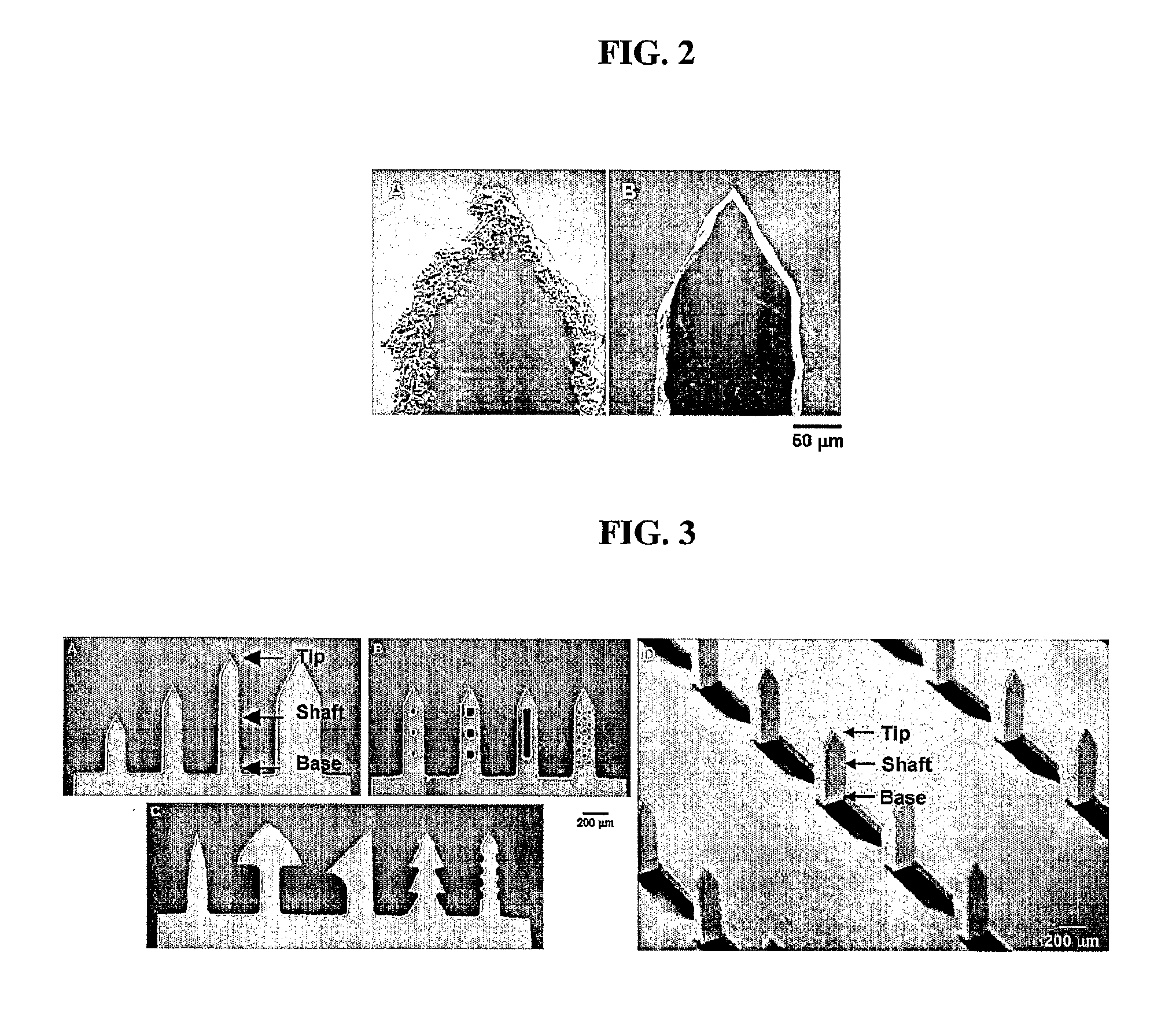
[0120]Microneedles were also made with a variety of shapes in increasingly complex geometries using laser etching. First, microneedles of different lengths and widths with a constant tip angle of 55° were created (FIG. 3A). Next, microneedles were made with small through-holes (i.e., “pockets”) of different shapes and sizes in the shafts of the ...
example 2
Assembly of Coated Microneedle Patches
[0130]Coated microneedle arrays, made as in Example 1, were assembled into transdermal patches containing pressure-sensitive adhesive to adhere to the skin. To secure microneedles in the skin at all times until ready to be removed, microneedles were integrated into a Band-Aid-like patch. This patch had pressure-sensitive adhesive on one complete side, with microneedles protruding therefrom. The adhesive secured the microneedles and compensated for the recoiling tendency of the skin and the rigid stainless steel substrate of out-of-plane microneedles. These patches were fabricated using either multiple linear rows of in-plane microneedles or individual arrays of out-of-plane microneedles.
[0131]Microneedle Patches Using Multiple Rows of Microneedles
[0132]In-plane microneedles were fabricated with a uniform adhesive layer in between the microneedles. In this example, a set of ten rows of microneedles, containing five microneedles each, was assemble...
example 3
In Vitro Dissolution of Microneedle Coating
[0135]To assess the in vitro dissolution time, single microneedles (n=3) coated with vitamin B, calcein, or sulforhodamine, made as in Example 1, were inserted into pig cadaver skin for 10 s or 20 s. Upon removal, these microneedles were imaged by fluorescence microscopy to detect residual coating. After 10 s insertion, a majority of the coating was dissolved. After 20 s insertion, the microneedle coating was completely dissolved. A sulforhodamine-coated microneedle showed similar dissolution and release into skin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com