Methods and compositions for treating meibomian gland disease
a meibomian gland and composition technology, applied in the direction of drug compositions, aerosol delivery, extracellular fluid disorder, etc., can solve the problems of meibomian gland atrophy, short treatment period, and potential sight-threatening problems, so as to reduce ocular irritation, delay the effect of tear clearance and relief of symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Increased IL-1α Concentrations in Meibomian Gland Disease Patients
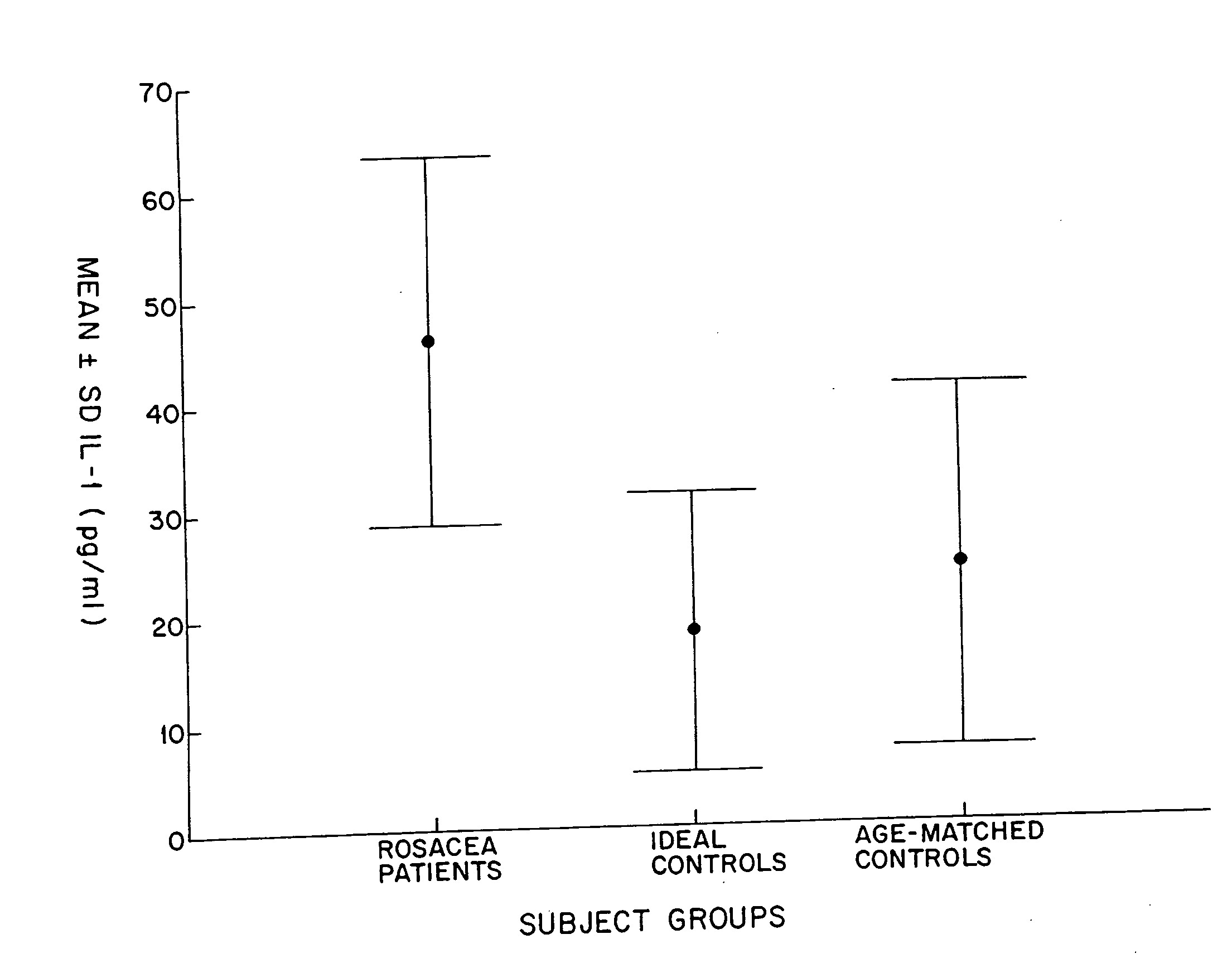
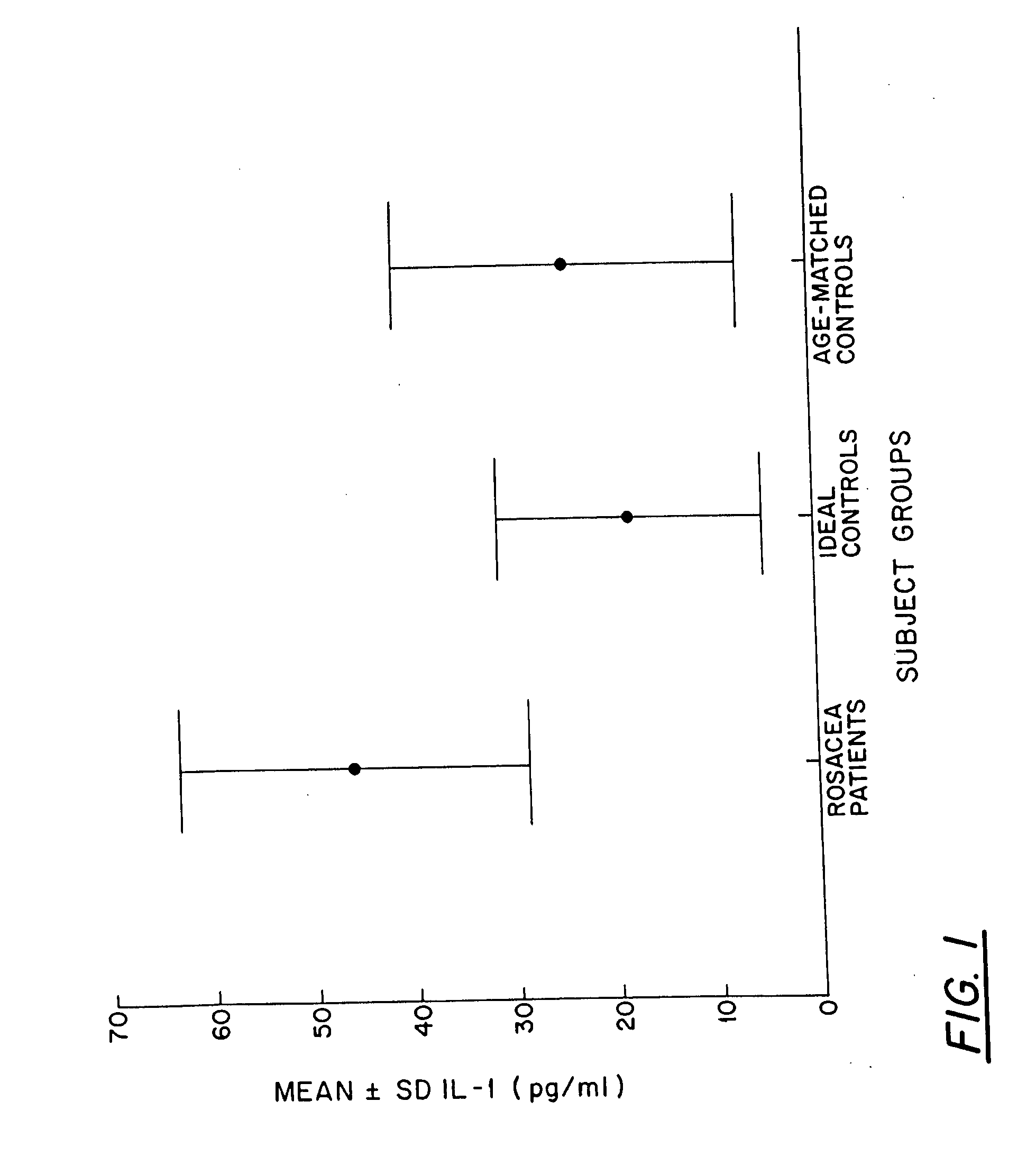
[0034]In one study, tear fluid concentrations of Interleukin-1-alpha (IL-1α), Tumor Necrosis Factor-α (TNF-α), and Epidermal Growth Factor (EGF) in patients having ocular rosacea were compared with those concentrations in normal patients (controls).
[0035]Fourteen (14) patients with severe meibomian gland disease, facial rosacea, and symptoms of ocular irritation were examined for ocular surface disease, tear production and tear clearance rate (TCR). For meibomian gland disease assessment, meibomian glands were examined by slit lamp biomicroscopy and graded for the presence of orifice metaplasia, expressibility of meibum, and meibomian gland acinar dropout, as previously described and known from the scientific literature. Twelve (12) controls, frequency-matched for age, and fifteen (15) ideal normals were assessed using the same parameters.
[0036]Minimally stimulated tear samples (20 μl) were drawn fro...
example 2
Correlation of Gelatinise Activity with IL-1α Concentration and Tear Clearance
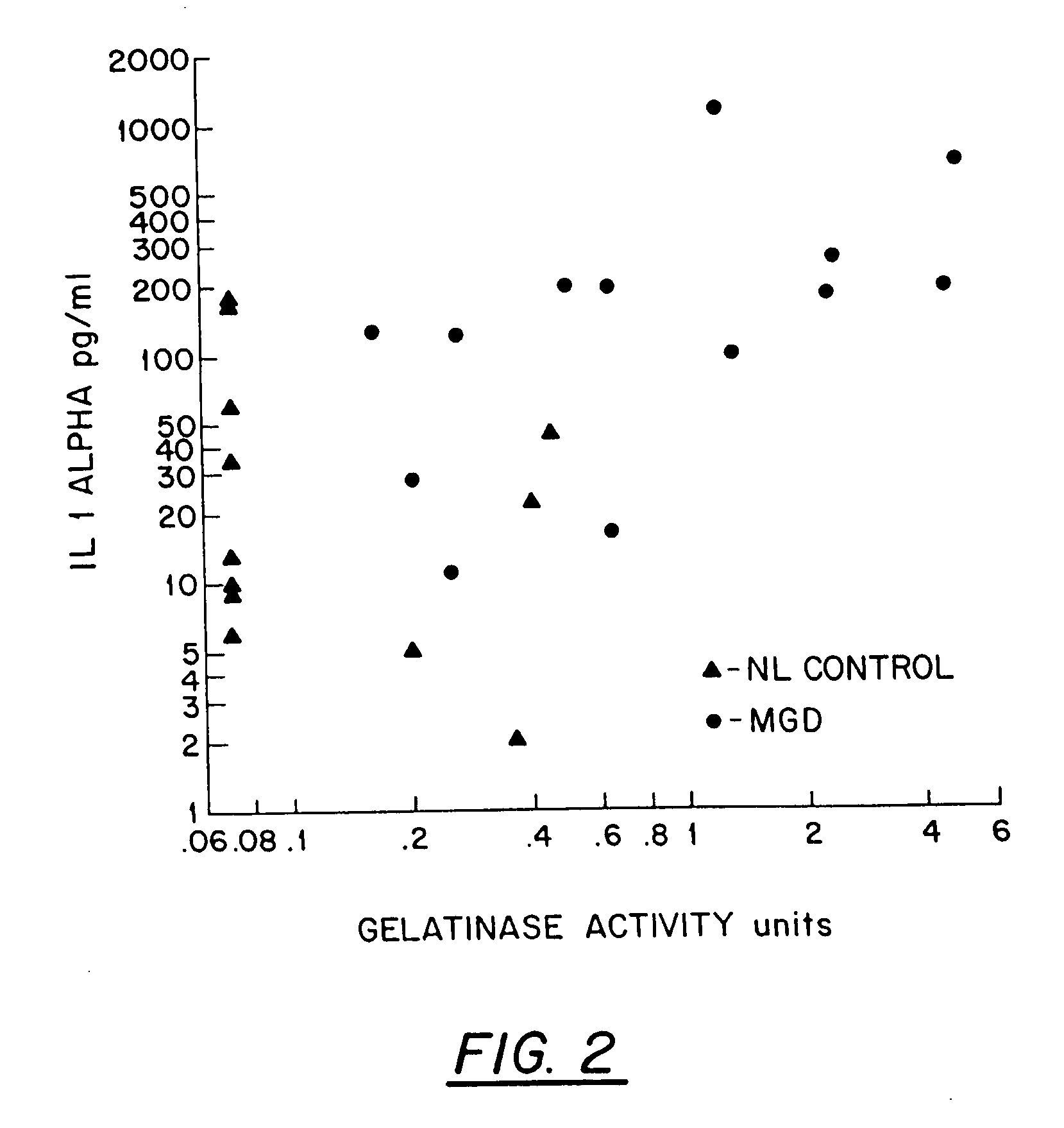
[0041]Tear fluorescein clearance was correlated with IL-1α concentration and 92 kD gelatinase (MMP 9) activity in the tears of patients. Thirteen patients with ocular rosacea (including 1 patient with recurrent epithelial erosion, 2 with recurrent peripheral corneal infiltrates and vascularization and 2 patients with epithelial basement membrane dystrophy) and 13 normal subjects with normal aqueous tear production and no irritation symptoms were evaluated. Tear fluorescein clearance was evaluated by measuring fluorescence in tear fluid collected from the inferior meniscus 15 minutes after instillation of 5 ml of 2% Na-fluorescein with a Cytofluor II. IL-1α was measured by ELISA using an R&D Systems kit. Gelatinase activity was evaluated by gelatin zymography, comparing tear activity to purified 92 kD gelatinase (MMP 9).
[0042]Compared to normal controls, patients with ocular rosacea had greater delay of flu...
example 3
Reduced Tear Clearance in Ocular Irritation
[0043]Reduced tear clearance is commonly found in most patients with ocular irritation irrespective of the patient's tear production. Forty (40) abnormal patients presenting with a chief complaint of ocular irritation and forty (40) asymptomatic controls of similar age distribution were used to correlate and compare a new method of measuring tear fluorescein clearance and the Schirmer 1 test with the severity of ocular irritation symptoms, presence of meibomian gland disease, corneal fluorescein staining scores, and corneal and conjunctival sensitivity. All subjects completed a symptom questionnaire, a baseline ocular examination, fluorescein clearance test (FCT) and Schirmer test.
[0044]Methods. The fluorescein clearance test (FCT) was performed by measuring the fluorescein concentration in minimally-stimulated tear samples collected from the inferior tear meniscus 15 minutes after instillation of 5 μl of 2% sodium fluorescein with a Cytofl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com