Combination therapy with a compound acting as a platelet adp receptor inhibitor
a technology of platelet adp receptor and compound, which is applied in the direction of drug compositions, peptides, cardiovascular disorders, etc., can solve the problems of thrombotic complications and vascular occlusion, and achieve the effect of improving safety, improving therapeutic results, and reducing the amount to achieve equivalent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Compound A and Its Potassium Salt Formula I
[0214]
Step 1:
[0215]Methyl 2-amino-4,5-difluorobenzoate (1) (38 kg, 1.0 eq.) and dichloromethane (560 kg, 8×, ACS>99.5%) were charged to a 2000 L GL reactor. The reaction mixture was agitated for mins. 4-Nitrophenylchloroformate (49.1 kg, 1.2 eq.) was charged into the 200 L reactor followed by dichloromethane (185 kg) and the contents were agitated for 5 mins. After pressurizing the 200 L reactor, the 4-nitrophenylchloroformate solution was transferred into a 2000 L reactor containing dichloromethane solution of compound 1. The reaction mixture was heated to 40±5° C. (reflux) under nitrogen gas purge for 3 hrs. Representative TLC analysis confirmed completion of reaction (in-process TLC, no compound 1 remaining; 99:1 CHCl3-MeOH). The solution was cooled to 30° C. and 460 kg of dichloromethane was distilled off under vacuum. The 2000 L reactor was charged with 520 kg of hexanes and the contents of the reactor were cooled to 0±5...
example 2
Preparation of Betrixaban
[0223]
Step 1:
[0224]5-Methoxy-2-nitrobenzoic acid (5) (25.0 kg, 1.0 eq.), 2-amino-5-chloropyridine (6) (16.3 kg, 1.0 eq.), and acetonitrile (87.5 kg, 3.5 parts) were charged to a 380 L GLMS reactor. The reaction mixture was adjusted to 22° C. (19 to 25° C.) and anhydrous pyridine (30.0 kg, 3.0 eq.) was added. The pump and lines were rinsed forward with acetonitrile (22.5 kg, 0.9 parts), and the reactor contents were adjusted to a temperature of 19-22° C. Phosphorous oxychloride (23.3 kg, 1.20 eq.) was charged to the contents of the reactor via a metering pump, while maintaining a temperature of 25° C. (22-28° C.). The metering pump and lines were rinsed forward with acetonitrile (12.5 kg, 0.5 parts), while keeping the temperature at 25° C. (22-28° C.). The reaction mixture normally turned from a slurry to a clear solution after the addition of about ⅓ of the POCl3. At the end of the addition, it became turbid. After complete addition, the reaction mixture was...
example 3
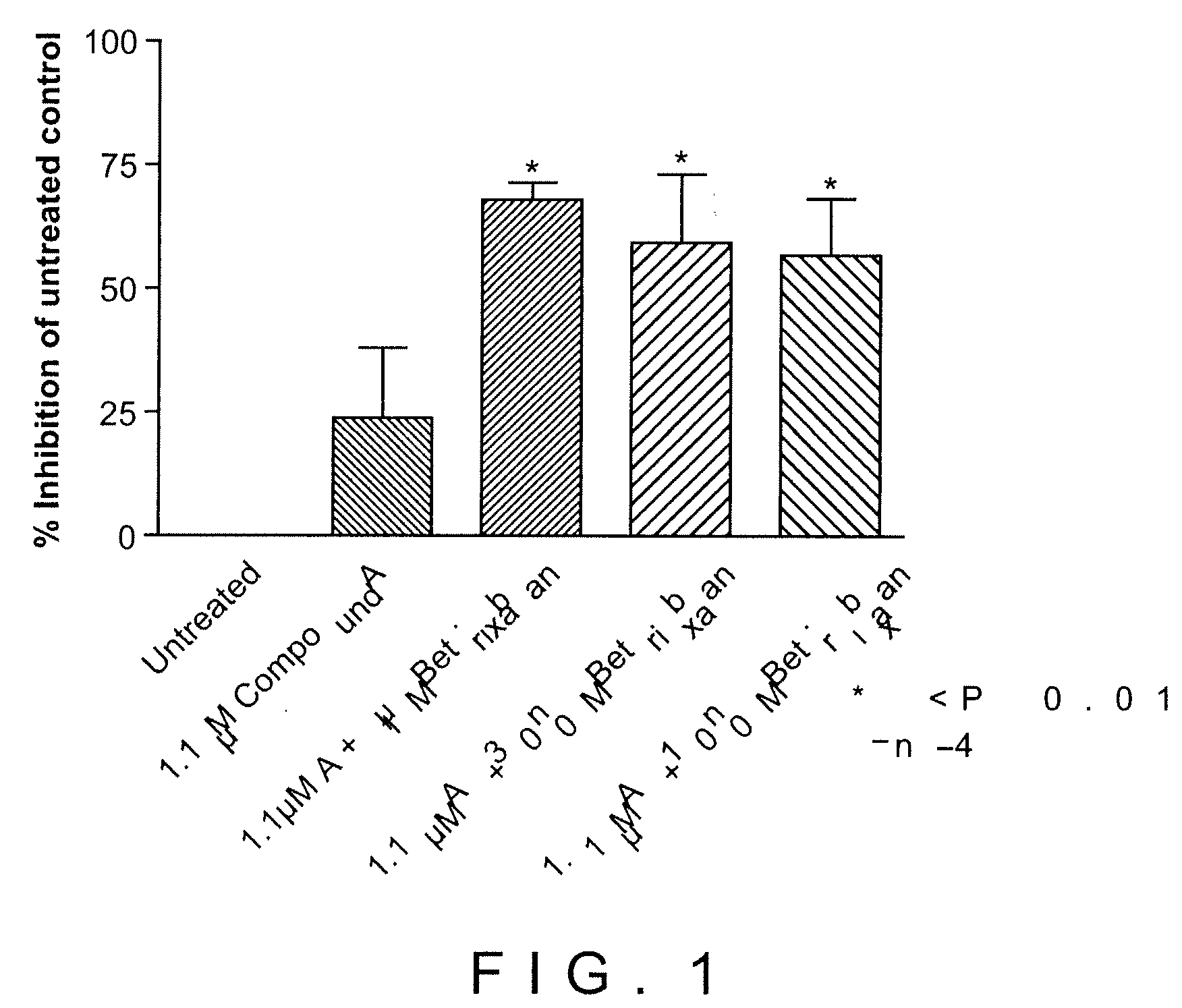
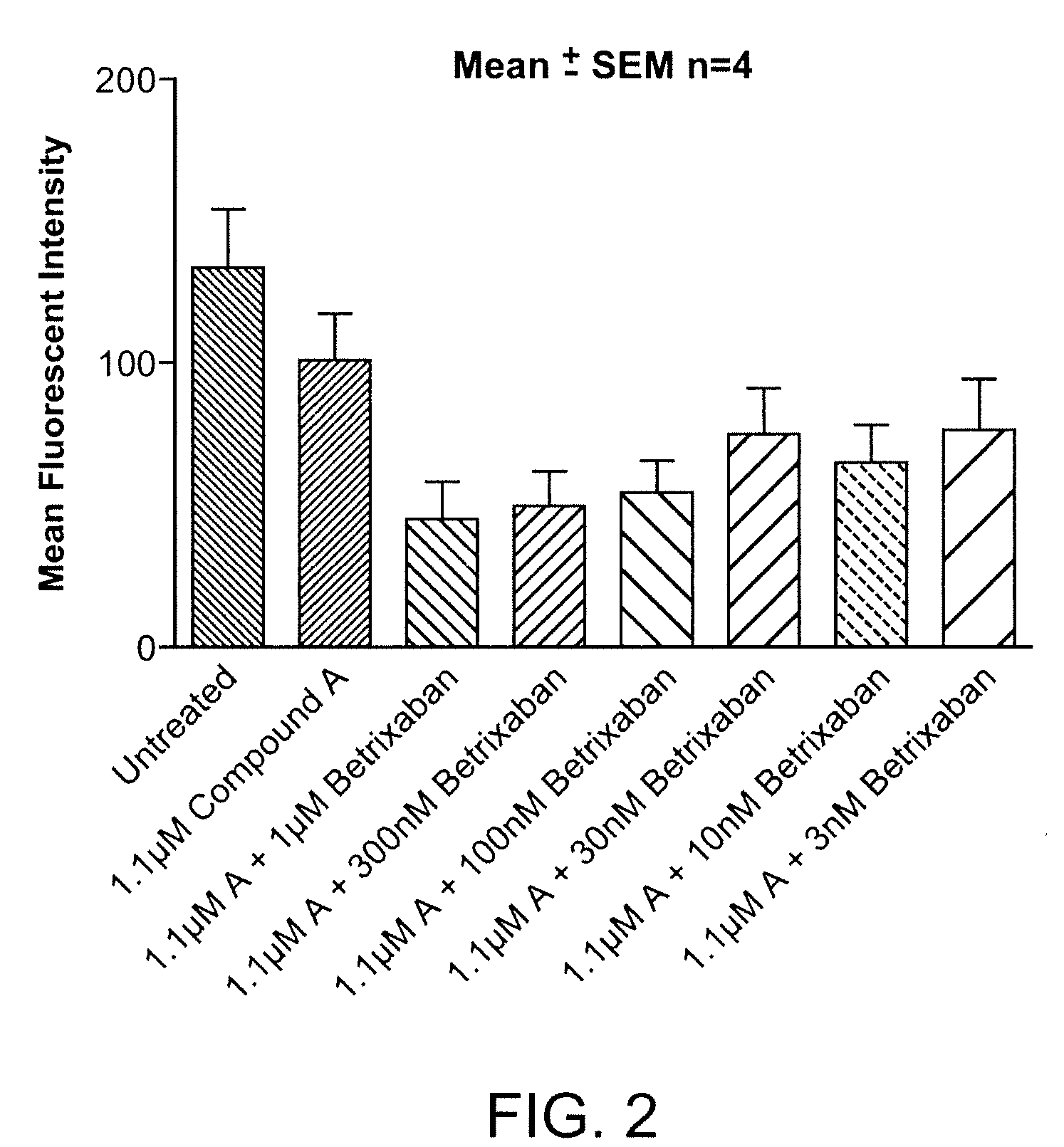
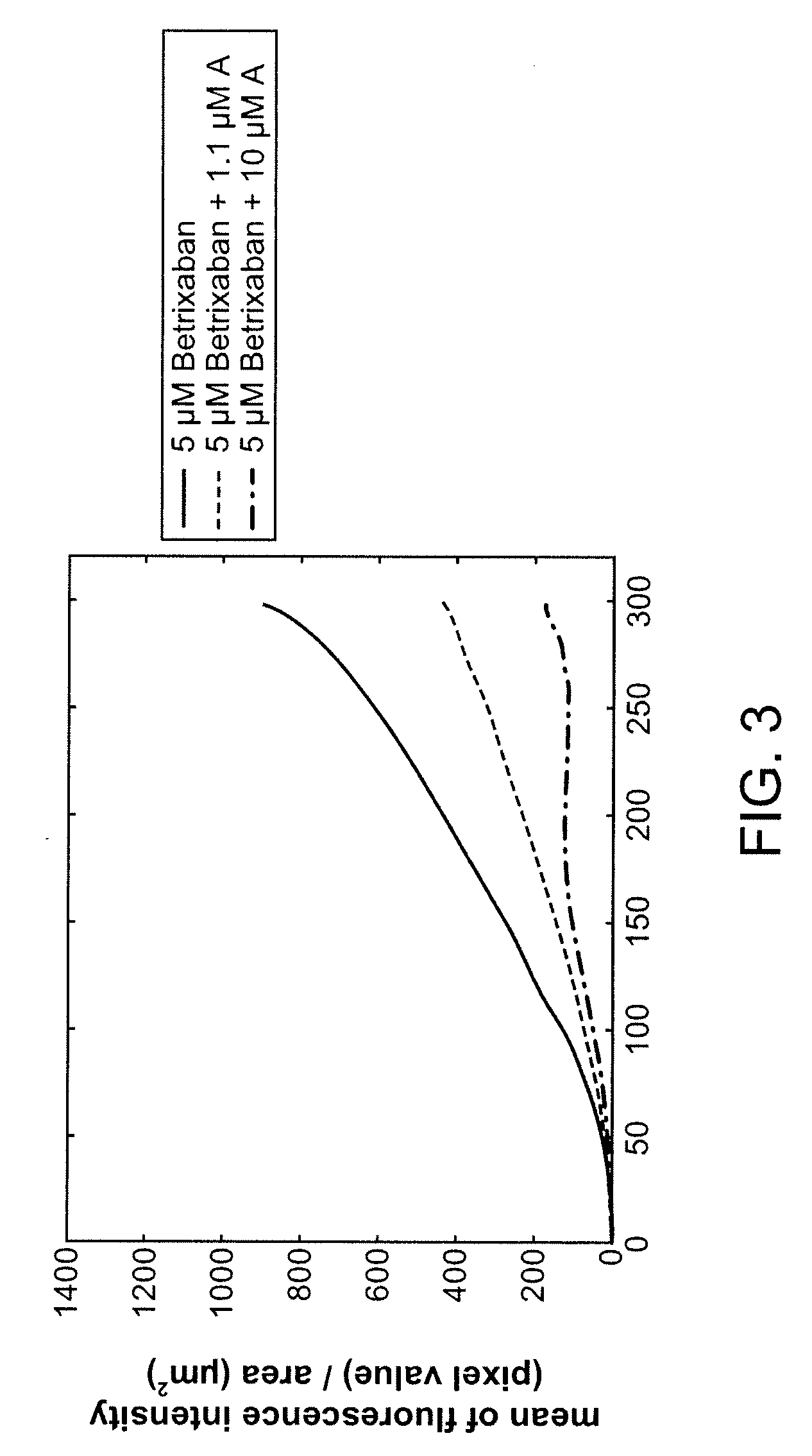
Combination of Compound A and Betrixaban in a Perfusion Chamber Thrombosis Assay (I)
[0228]The real time perfusion chamber assay couples the features of animal thrombosis models that use intravital microscopy to those of perfusion chamber technology in order to produce an assay suited to monitoring drug activity in clinical trials. This assay perfuses whole blood through capillaries at arterial rates of shear, exposing the blood to thrombogenic type III collagen. Platelets are labeled with a fluorescent dye (rhodamine 6 G) prior to perfusion such that analysis of the thrombus deposition can be performed by measurement of fluorescence intensity inside the perfusion chamber. Quantification is performed by analysis of the thrombus height (fluorescence intensity (number of pixels) / total area (μm2)).
[0229]Compound A was tested in this assay at a concentration capable of generating equivalent levels of inhibition of ADP induced platelet aggregation in human platelet rich plasma as targeted...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| 2θ | aaaaa | aaaaa |
| 2θ | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com