Carbon-Encased Metal Nanoparticles and Sponges, Methods of Synthesis, and Methods of Use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0102]The surface of the cellulose fiber is rough (FIG. 3A) and contains pores of diameter of 30-70 nm (FIG. 3B). These nanopores may allow reactant molecules to penetrate into inner cavities. When cellulose fibers were immersed in aqueous CuSO4, copper ions were readily impregnated into the cellulose fibers through the pores. Though not wishing to be bound by this theory, most of the incorporated Cu++ ions appeared to be bound to cellulose macromolecules, probably via electrostatic (e.g., ion-dipole) interactions, with the electron-rich oxygen atoms of polar hydroxyl and ether groups of cellulose.
example 2
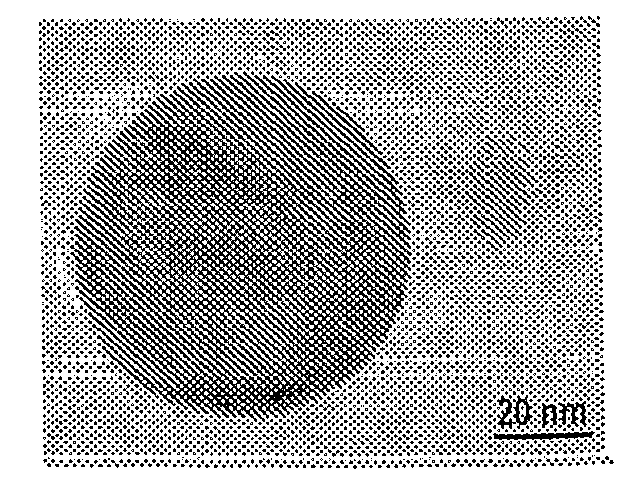
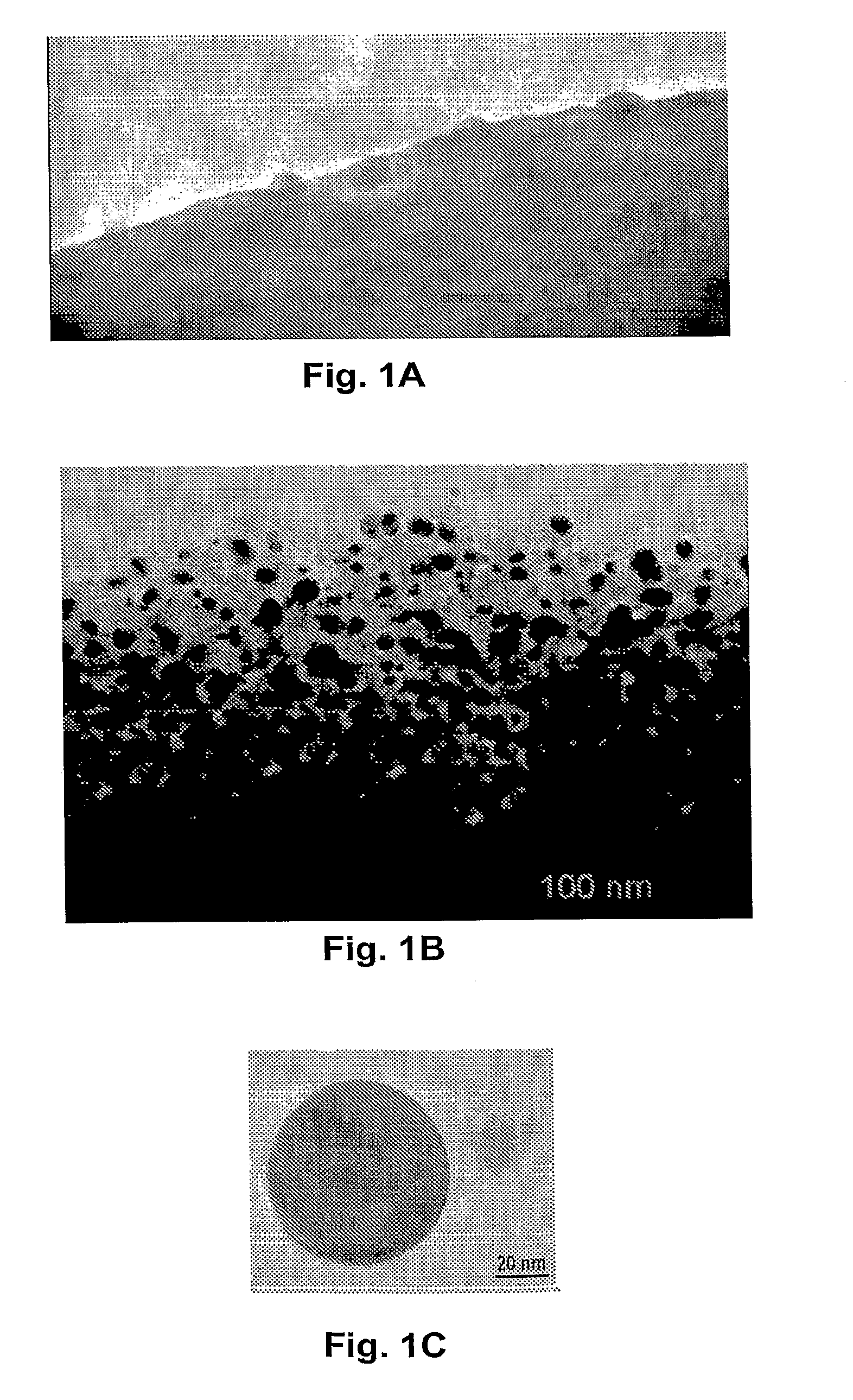
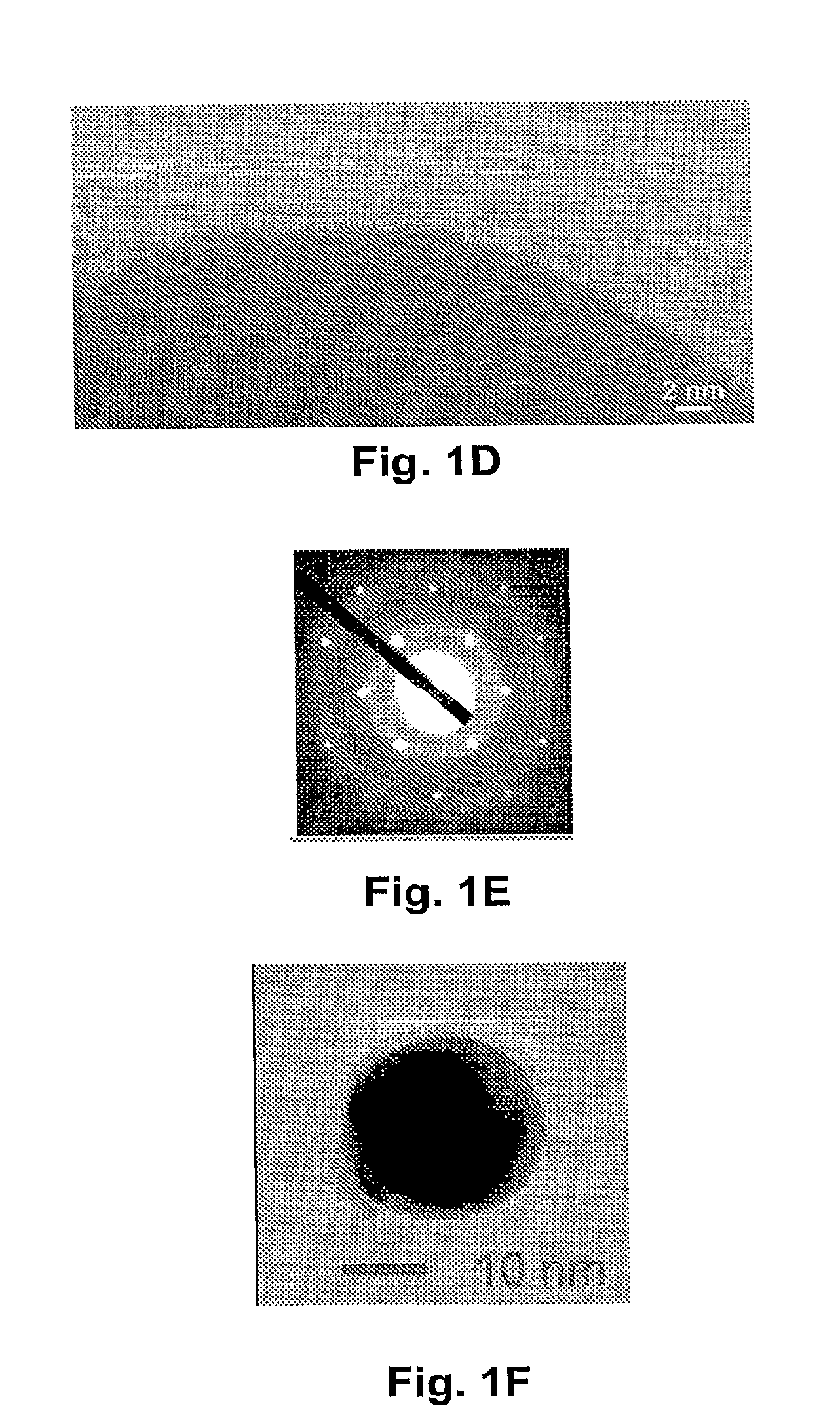
[0103]Cotton fiber was soaked in a copper sulfate solution. After the cotton was saturated, then extra solvent was removed. Carbonization was carried out at about 350° C. under nitrogen for about two hours. The copper nanoparticles and the encapsulating carbon shells appeared to have been formed simultaneously during carbonization. As fabricated, the CCCSNPs were uniformly distributed throughout the carbon based matrixes. FIGS. 1A, 1B, 1C, 1D, and 1F depict micrographs of fabricated copper-carbon core-shell nanoparticles made through the present invention. FIG. 1A depicts an SEM image that shows the carbonized cotton fiber (carbon black) with many nanoparticles located on its surface; FIG. 1F depicts a higher magnification of that depicted in FIG. 1A. FIG. 1B depicts a TEM image demonstrating that the nanoparticles formed through the entire carbonized cotton fiber; FIGS. 1C and 1D depict TEM micrographs of a nanoparticle encased in a carbon shell. These micrographs show a core of ab...
example 3
[0104]The total copper concentration in the material made according to Example 2 was measured to be about 25 Wt %. It was clear from the TEM micrograph (See FIG. 1B) that the particles had generally spherical shapes, and that a majority of them appeared to have an average diameter value about 20-50 nm, although some particles were as small as one or two nanometers in diameter.
[0105]After the carbonized material was pulverized into micrometer to sub-micrometer sized particles, it was uniformly dispersed into both polar and non-polar solvent, for example water, aqueous acids, aqueous bases, salt solutions and cooking oil. After being immersed in water at ambient environment over three months, the nanoparticles still retained a reduced copper core structure with no sign of deterioration. The powder, characterized by FTIR, also showed that the shells of the CCCSN particles retained a number of organic functional groups. This property will be useful in functionalizing the carbon layer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com