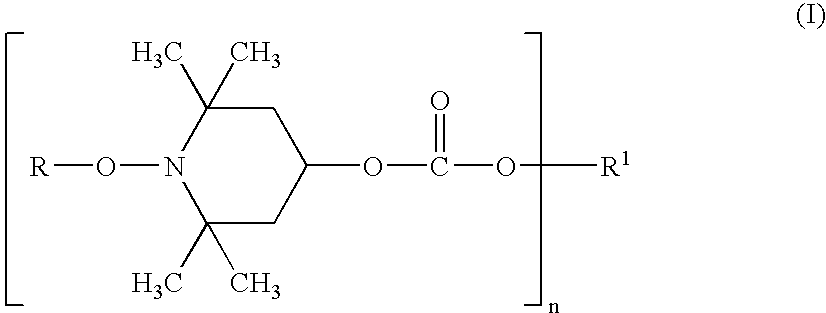
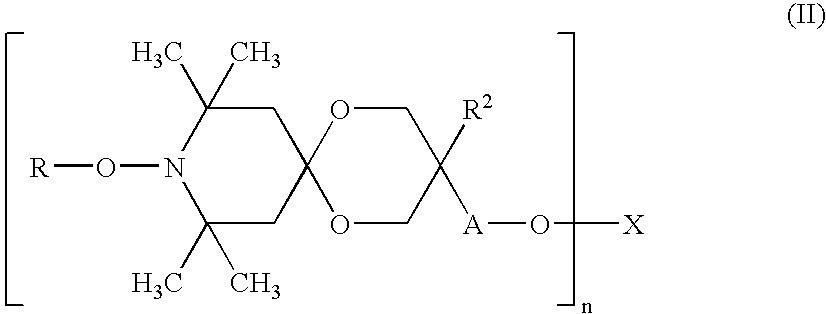
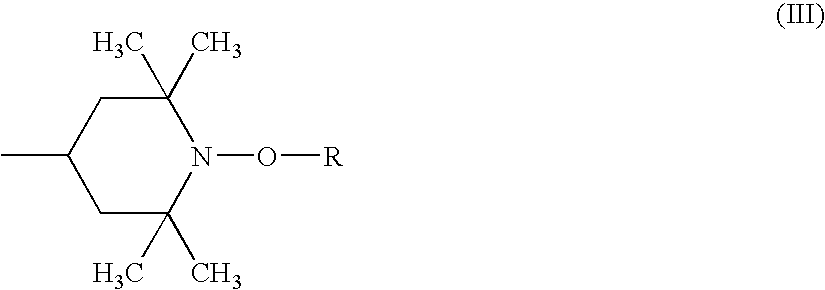Weakly Basic Hindered Amines Having Carbonate Skeletons, Synthetic Resin Compositions, And Coating Compositions
a technology of hindered amines and carbonates, which is applied in the field of weakly basic hindered amine compounds having carbonate skeletons, can solve the problems of low compatibility, inability to sustain the stabilization effect, and extraction by acid rain, and achieves long-term stabilization and superior resistance to acid rain extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compound No. 1
[0071]17.0 g (98.1 mmol) of 4-hydroxy-1-oxy-2,2,6,6-tetramethyl piperidine was dissolved in 40.0 g of chlorobenzene, and a solution containing 31.3 g (78.5 mmol) dilauroyl peroxide dissolved in 125 g chlorobenzene was dripped in at 70° C. over 3 hours. The reaction was performed at this temperature for a further 6 hours. The obtained reaction liquid was analyzed by gas chromatography to verify consumption of the starting materials. The obtained reaction liquid was a mixture of 4-hydroxy-1-undecanoxy-2,2,6,6-tetramethyl piperidine, 1-undecanoxy-2,2,6,6-tetramethyl piperidine-4-one, lauric acid and a solvent. 50 g of hexane was added to the reaction liquor, the reaction liquor was washed with 53.9 g (98 mmol) of 7.3% sodium hydroxide aqueous solution and 25 g methanol, washed twice more with 30 g water, and lauric acid was removed. The mixture was dried with anhydrous magnesium sullfate, the magnesium sulfate was removed by filtration, and the solvent was re...
example 2
Synthesis of Compound No. 7
[0074](Synthesis of 1-undecaneoxy-2,2,6,6-tetramethyl piperidine-4-one)
[0075]15.0 g (86.6 mmol) of 4-hydroxy-1-oxy-2,2,6,6-tetramethyl piperidine was dissolved in 40.0 g of chlorobenzene, and a solution containing 27.6 g (69.3 mmol) dilauroyl peroxide dissolved in 125 g chlorobenzene was dripped in at 70° C. over 3 hours. The reaction was performed at this temperature for a further 6 hours. The obtained reaction liquid was analyzed by gas chromatography to verify consumption of the starting materials. 0.1 g of 4-acetyl-1-oxy-2,2,6,6-tetramethyl piperidine was added to the reaction liquor to suppress decomposition reactions, the mixture was cooled to 0° C., and 48.3 g (64.9 mmol) of 10% sodium hypochlorite aqueous solution was dripped in over 3 hours. The reaction was continued for 3 hours at the same temperature, 15 ml of 15 wt % sodium thiosulfate aqueous solution was added, and the mixture was heated to 40° C. and reacted for 1 hour. The organic layer an...
example 3
Synthesis of Compound No. 11
[0080]8.0 g (20 mmol) of the 1,5-dioxa-9-aza-3-hydroxy-8,8,10,10-tetramethyl-9-undecyloxyspiro[5.5]und ecane obtained in Synthesis Example 2, 2.35 g (11.0 mmol) diphenyl carbonate and 0.7 g potassium carbonate were dispersed in 100 ml mineral spirit, reacted at 170-180° C. for 8 hours, and phenol was removed. The mixture was cooled to 40° C., and washed 3 times with 30 ml water. Water was removed under reduced pressure with reflux at 60° C., and the solvent was removed under reduced pressure on the evaporator. The concentrate was recrystallized from ethanol by cooling to 0° C., and Compound No. 11 of purity 99.9% was obtained as colorless crystals with a melting point of 87.4° C. (yield 68.4%).
[0081]The analysis result of the obtained compound No. 11 is shown below.
IR spectrum
2850-2920 cm−1, 1750 cm−1, 1470 cm−1, 1360 cm−1, 1280 cm−1, 1230 cm−1, 1200 cm−1, 1100 cm−1, 1030 cm−1, 960 cm−1.
1H-NMR spectrum (H: Actual measurement of number of protons, figures ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com