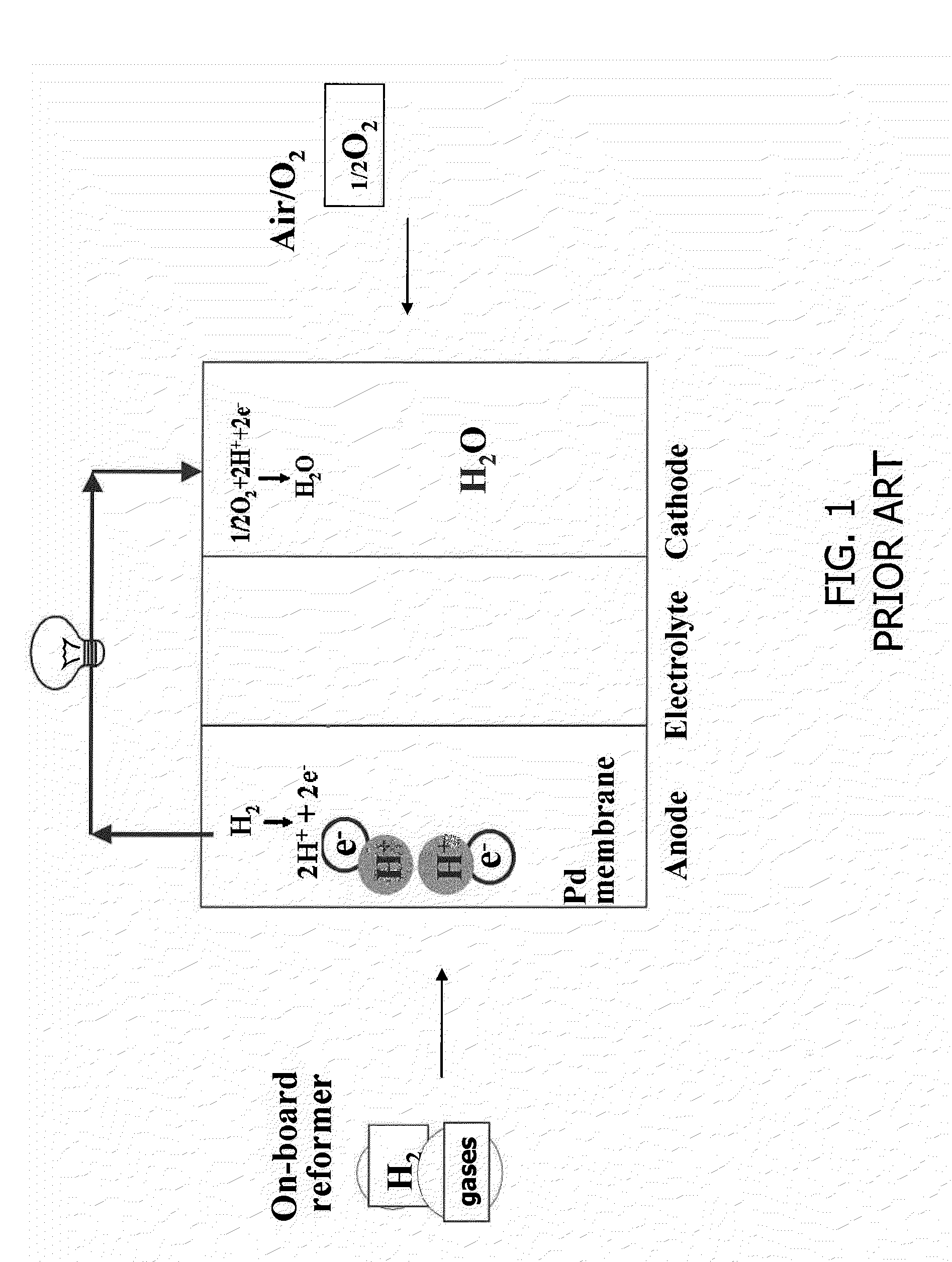
Ion-Conducting Ceramic Apparatus, Method, Fabrication, and Applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
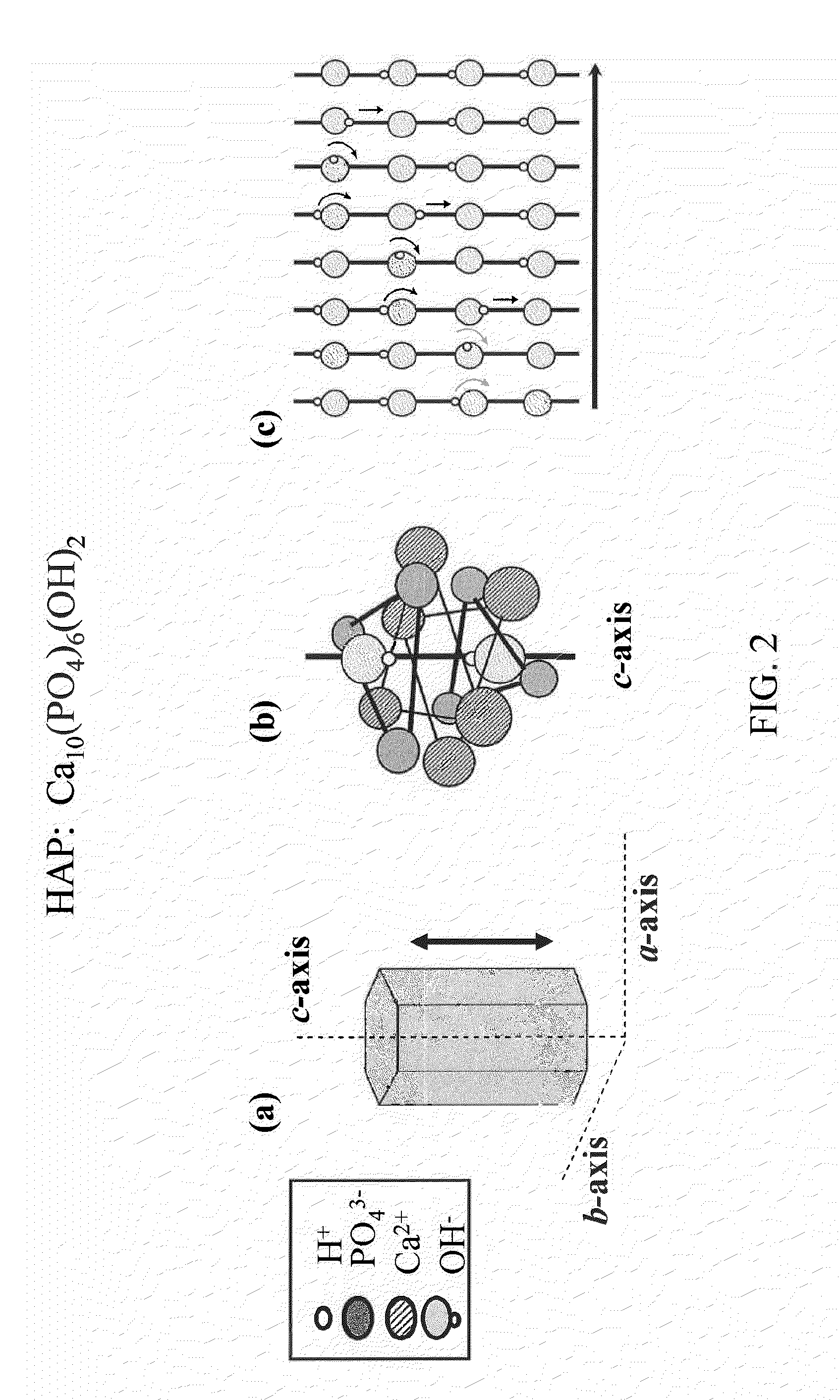
[0038]As mentioned above, optimal performance of a hydroxyapatite ion- / proton-conducting membrane occurs when the crystal domains span the entire thickness of the membrane to eliminate grain boundary resistance across the thickness of the membrane. In addition, the crystal's c-axis would be aligned so that the proton transport path is optimized, as illustrated in FIGS. 2(a-c).
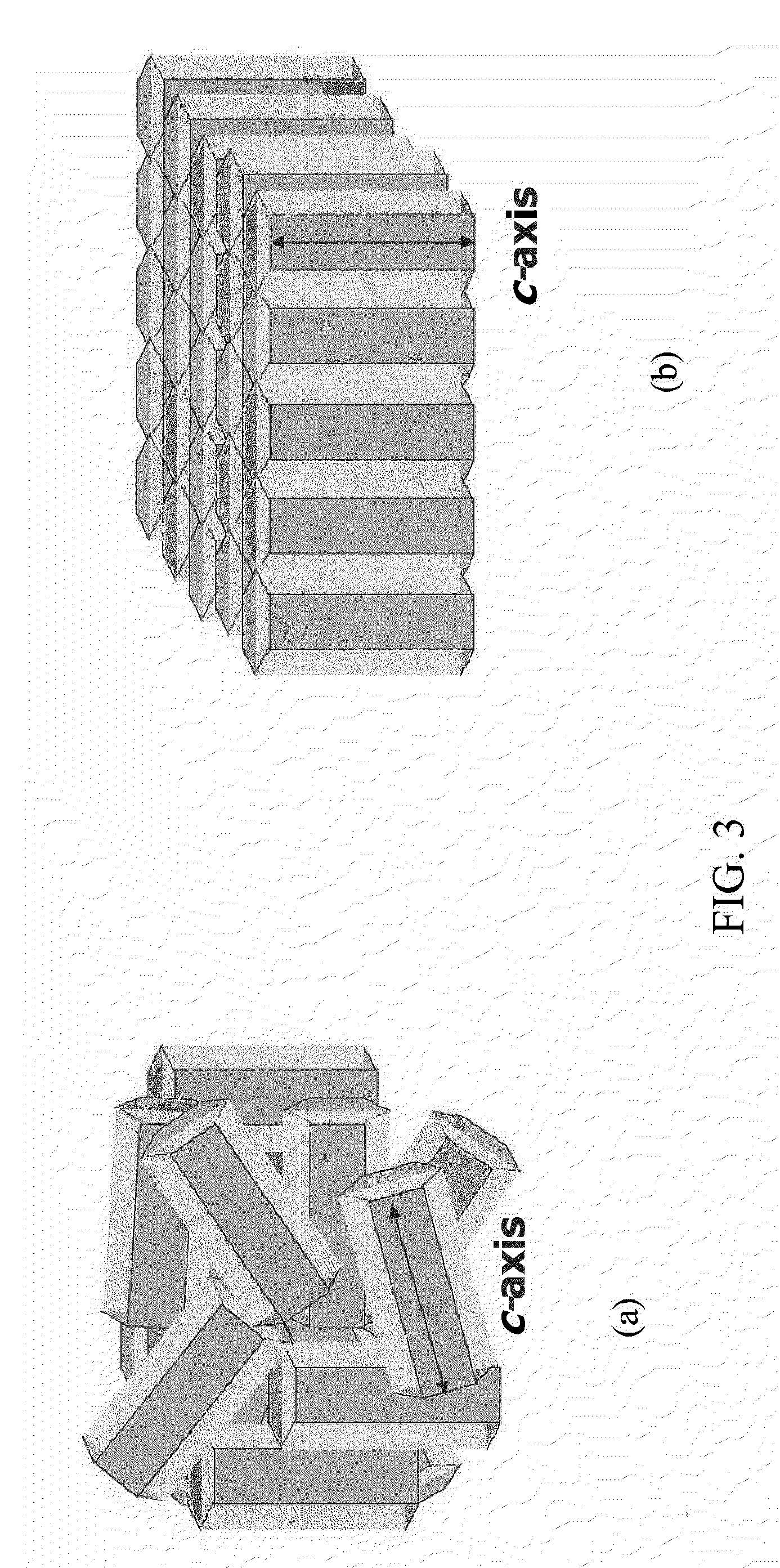
[0039]FIG. 3(a) schematically shows HAP crystals that are randomly oriented. FIG. 3(b), on the other hand, schematically shows an ideal HAP membrane structure with the c-axes of crystal domains spanning the entire membrane thickness to optimize proton transport;
[0040]A non-limiting, exemplary ion / proton conducting membrane 400-1 as illustrated in FIG. 4(c) includes a substrate 402 and a crystalline ion-conducting thin film 304 (also shown in FIG. 3(b)) having a thickness t. The thin film is characterized by a plurality of single apatite crystals 304′ each having its c-axis oriented normal to the substrate (as a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com