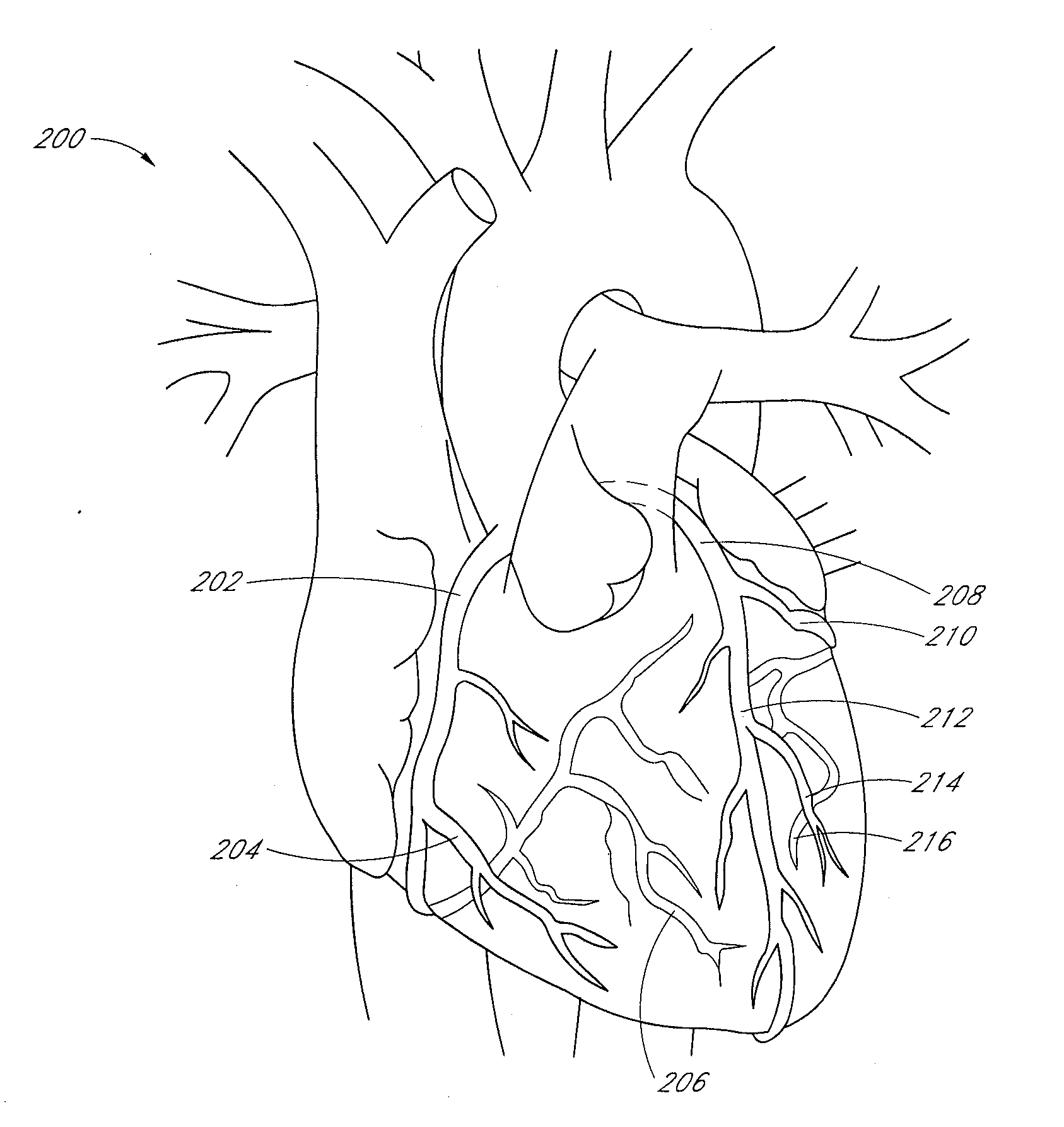
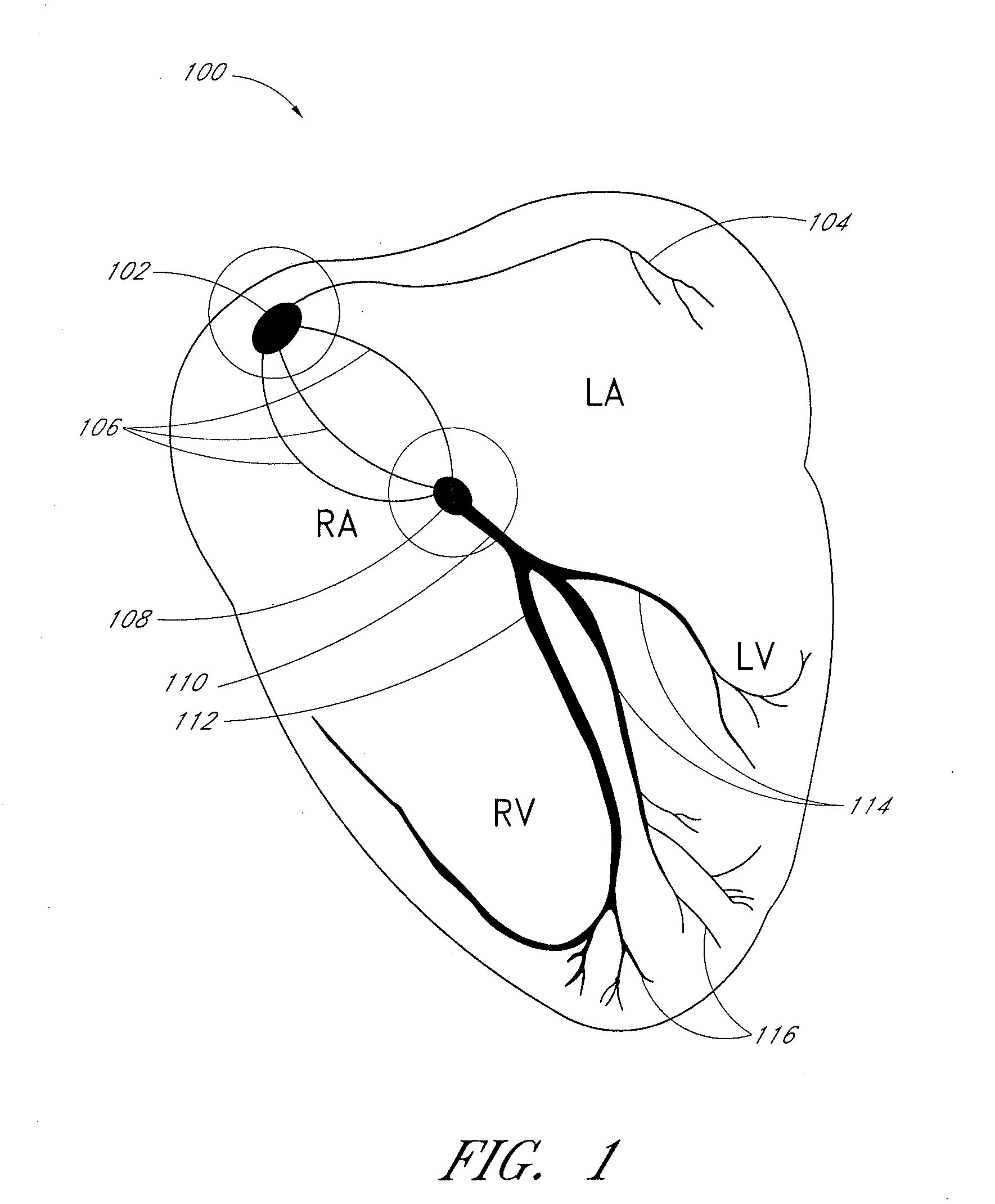
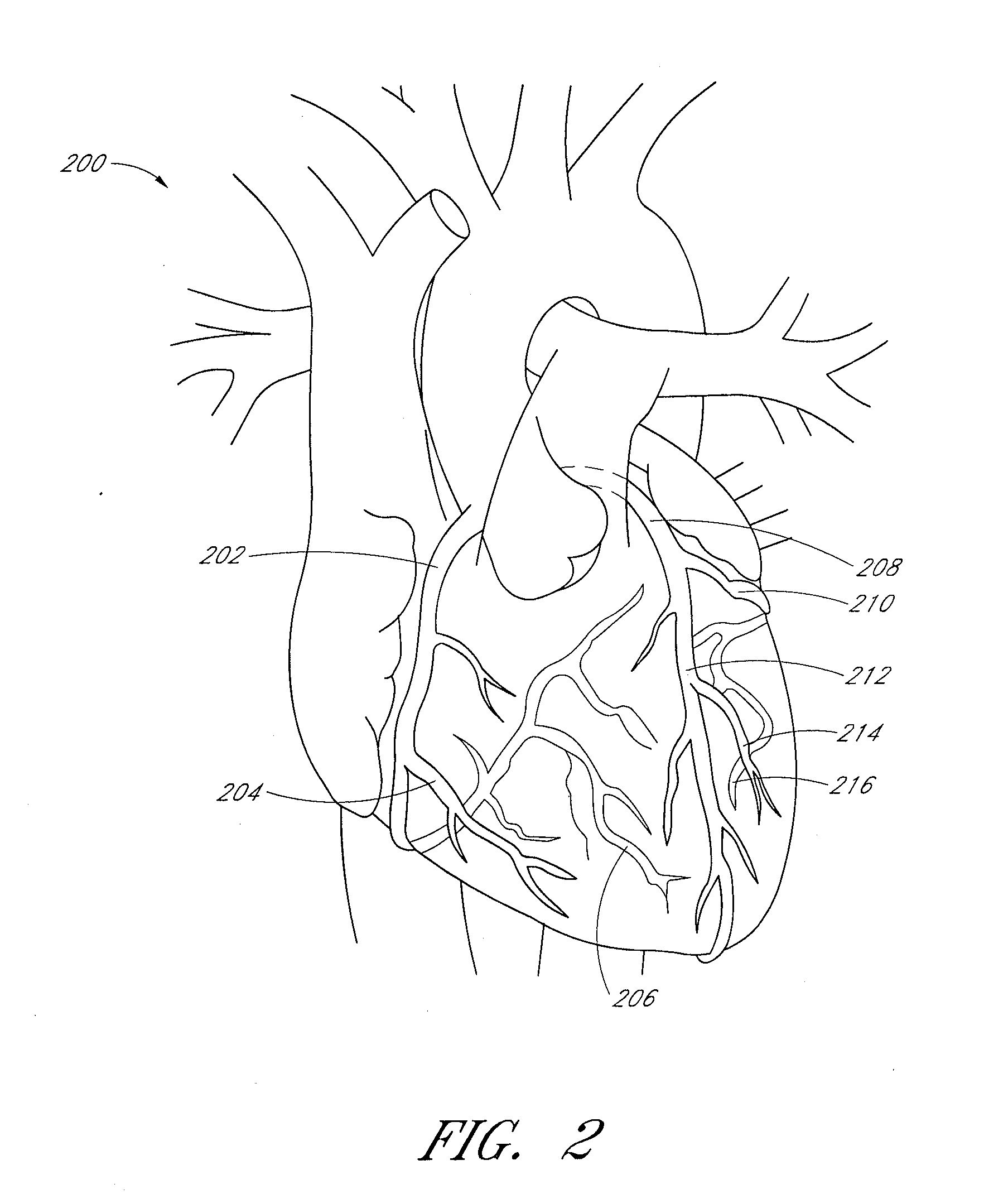
Use of platelet rich plasma composition in the treatment of cardiac conduction abnormalities
a technology of platelet rich plasma and cardiac conduction abnormality, which is applied in the field of treatment of cardiac conduction abnormalities, can solve the problems of reducing contractile efficiency, heart disease and stroke, and atrium contracting against a closed atrio-ventricular valv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0103]PRP was prepared using a centrifuge unit made by Harvest (Plymouth, Mass). (Similar units are available as The Biomet GPS system, the Depuy Symphony machine and the Medtronic Magellan machine.) Approximately 55 cc of blood was drawn from the patient using a standard sterile syringe, combined with 5 cc of a citrate dextrose solution for anticoagulation, and then spun down to isolate the platelets according to the manufacturer's protocol. These platelets were then resuspended in approximately 3 cc of plasma. The resulting platelet rich plasma solution (PRP) was quite acidic and was neutralized with using approximately 0.05 cc of an 8.4% sodium bicarbonate buffer per cc of PRP under sterile conditions to approximately physiologic pH of 7.4. The PRP was not activated through addition of exogenous activators. This PRP composition is referred to herein as autologous platelet extract (APEX).
example 2
[0104]Adult female mice (n=19) underwent left anterior descending (LAD) artery ligation (45 minutes) followed by 5 minutes reperfusion to mimic myocardial infarction. An APEX composition was prepared as described in Example 1 The extract was not activated through the addition of exogenous agent(s).
[0105]Unactivated PRP (n=10) or saline (n=9) was injected into murine myocardium. Three weeks later MRI was used to evaluate ejection fraction.
[0106]The data is shown in FIG. 1. PRP improves cardiac function by 28% (as measured by Left Ventricular Ejection Fraction) relative to control at 3 weeks after a heart attack. The data is statistically significant at p=0.04. This data supports the use of PRP in an ischemia-reperfusion heart model.
example 3
Treatment of a Reperfusion Arrhythmia in an Animal Model
[0107]Arrhythmias frequently occur when ischemic cardiac tissue is reperfused with blood. This can occur in a variety of situations; however the most common is following an acute myocardial infarction. Typically, when blood flow is successfully reestablished after a coronary artery has been blocked, reperfusion arrhythmias can occur. These arrhythmias are usually in the form of ventricular ectopy including, but not limited to, non-sustained ventricular tachycardia, sustained ventricular tachycardia and ventricular fibrillation. These arrhythmias can result in hemodynamic compromise and, in some cases, death.
[0108]The successful test of PRP in an animal model simulating a reperfusion arrhythmia following an acute myocardial infarction was conducted as follows: In a 40 Kg swine, the left anterior descending artery was identified using coronary angiography and then occluded with an angioplasty balloon (thus mimicking a myocardial ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com