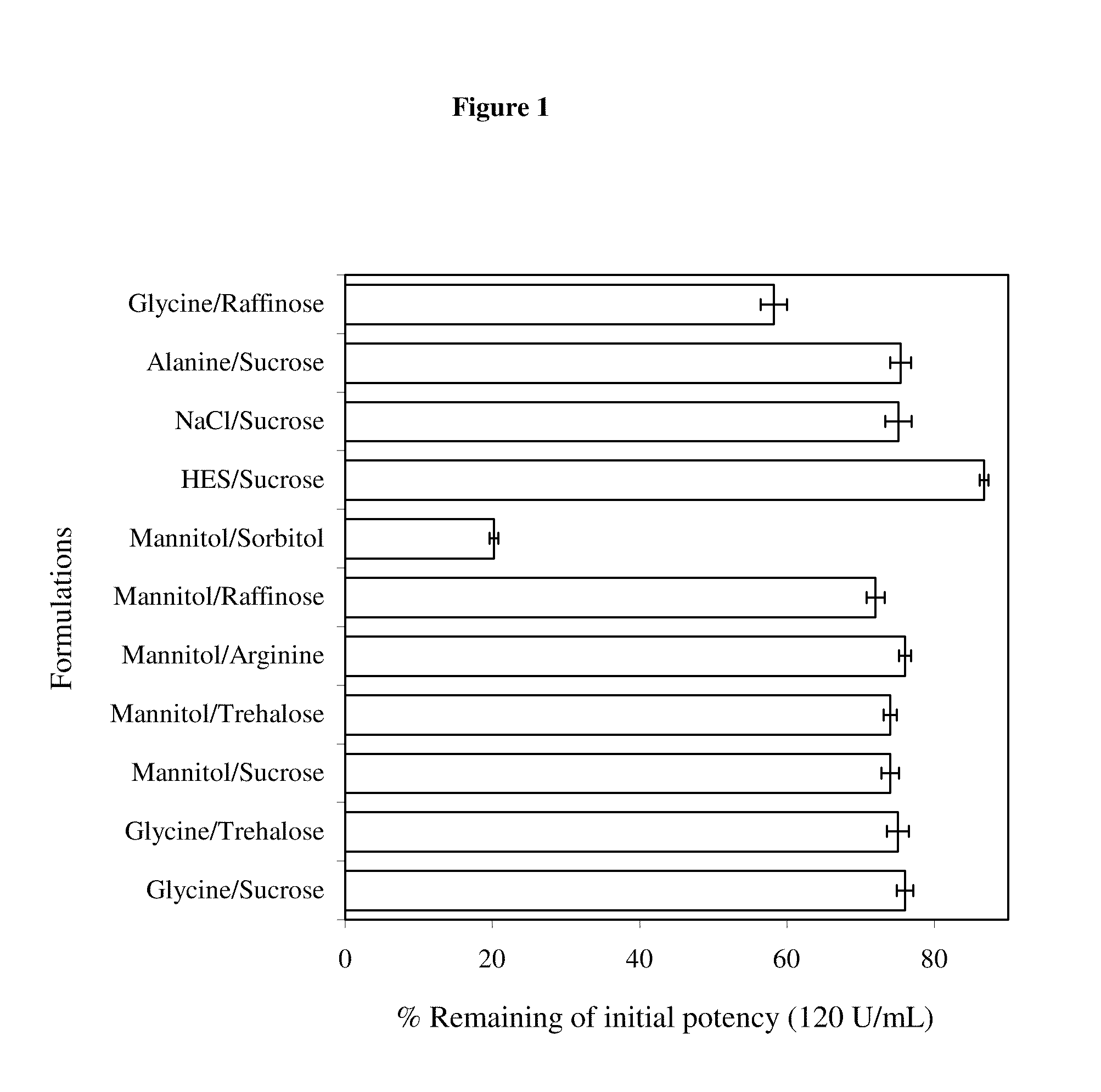
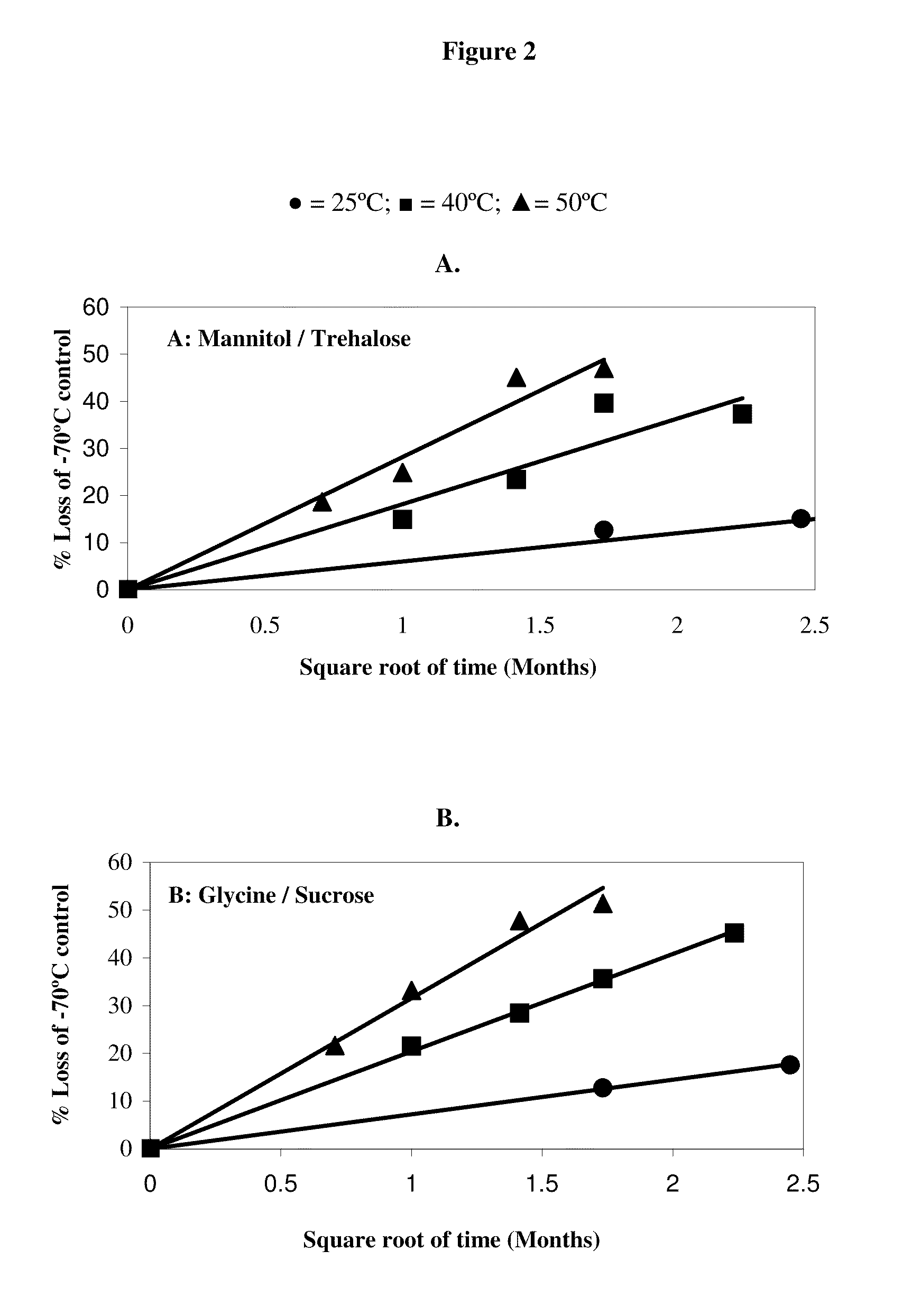
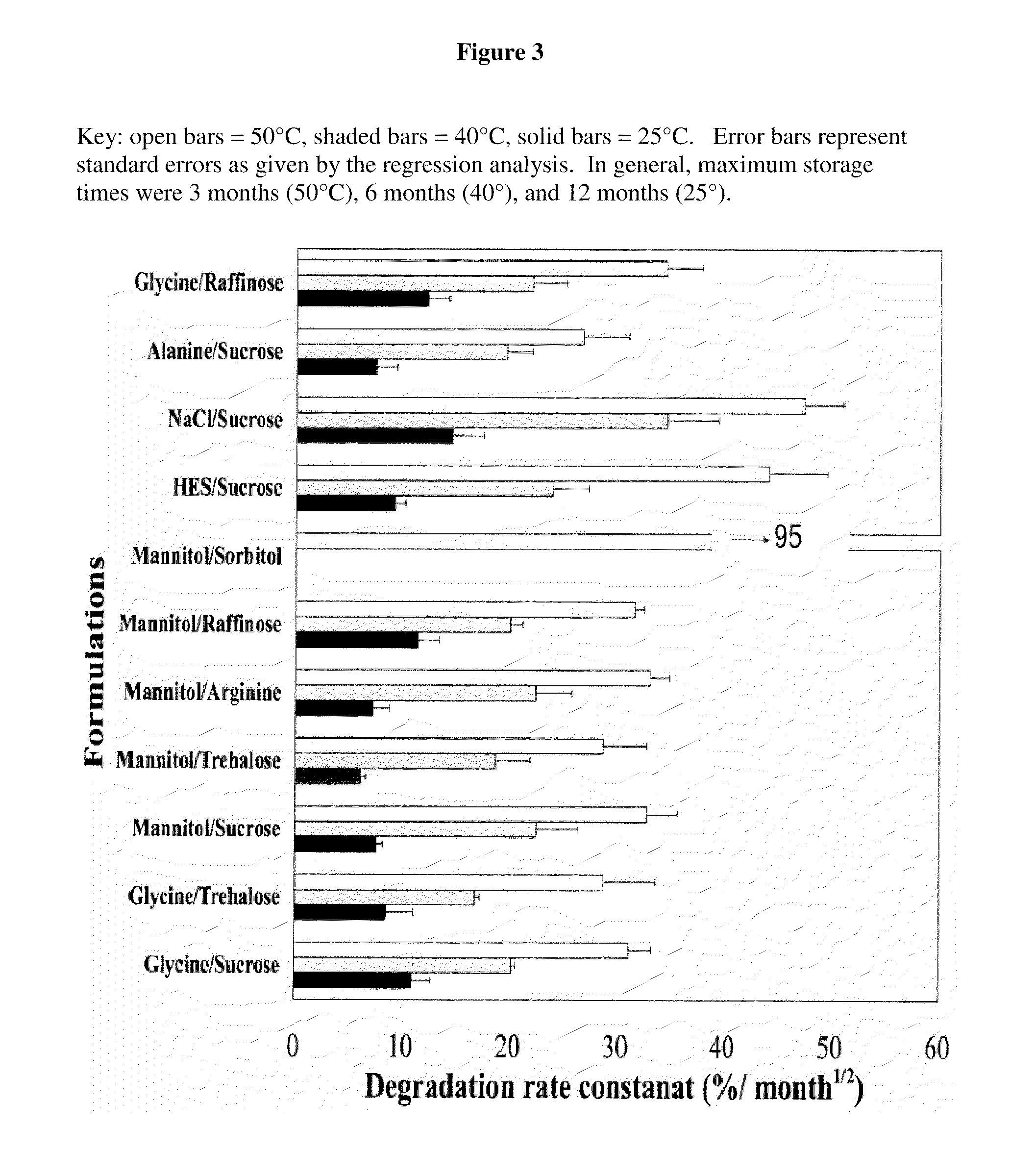
Factor viii formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Materials
[0117]The excipients used are analytical reagent grade. D-mannitol, trehalose, raffinose, sorbitol and sucrose were purchased from Pfanstiehl Lab. Inc. Glycine was obtained form Chattem Chemicals. M-hydroxyethyl starch, arginine, and alanine were obtained from Ajinomoto Co., Inc. Sodium chloride, calcium chloride and Tris base were obtained from Mallinckrodt Inc. Hepes, L-histidine and Polysorbate-80 were obtained from E. M. Science, Tanabe, Co. Inc. and Spectrum Int. Inc., respectively. Reduced glutathione was obtained from Sigma.
Formulation Preparation
[0118]The formulations described hereto were prepared using bulk drug substance (BDS), supplied by Baxter Healthcare Ltd. The BDS was composed of 3130 IU / ml rAHF (i.e., rFVIII); 50 mM Tris base, 0.4M NaCl, 0.1% polysorbate-80 (surfactant), 4 mM CaCl2, pH 7. The CaCl2 and surfactant are present to stabilize native conformation and reduce processing losses, respectively, and pH 7 was selected for optimal s...
example 2
[0125]Since variation in the level of NaCl is generally not acceptable, particularly in a commercial product, and since crystallization of a component is generally facilitated by increased concentration of that component relative to the other components, it was decided to add NaCl to increase the level to a value above the highest level expected in the process and sufficiently high so that crystallization of NaCl during the freezing step would be possible. Modulated Differential Scanning Calorimeter (MDSC) was used to study the freezing of various concentrations of sodium chloride in a formulation that further contained 0.02% (w / v) Polysorbate-80, mM Tris, 4 mM calcium chloride, and 2% (w / v) sucrose. For this experiment, 200 mM sodium chloride was used. Other concentrations could be used, including, but not limited to those in the range of 1 mM to 10000 mM, or 10 mM to 1000 mM or 100 mM to 500 mM, or 150 mM to 300 mM to ensure that complete crystallization of the sodium chloride occ...
example 3
[0127]The effect different concentrations of rAHF have on the stability of rAHF when the rAHF is exposed to stresses in different stages of freeze-drying was examined. Two samples containing different concentrations of rAHF, 600 and 60 IU / mL (corresponding to 150 & 15 μg / ml) were used without any stabilizer. The formulation composition for these samples was: 8% mannitol (bulking agent), 10 mM Tris buffer, 200 mM NaCl, 4 mM CaCl2, 0.02% Polysorbate-80, pH 7.0. A third sample used a formulation that employed 2% sucrose as a stabilizer in addition to these components. The drying process used, described in Table 1, produces product temperatures of about −42° C., which is sufficiently low to prevent collapse in most formulations; though this process was not optimized.
TABLE 1Freezing and lyophilization procedure for preliminary rAHFstability studies.Process stepDescriptionFreezingCool to +5° C., hold for 10 minutesCool to −5° C. at 1° C. / minute, hold for 20 minutesCool to −20° C. at 1° C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com