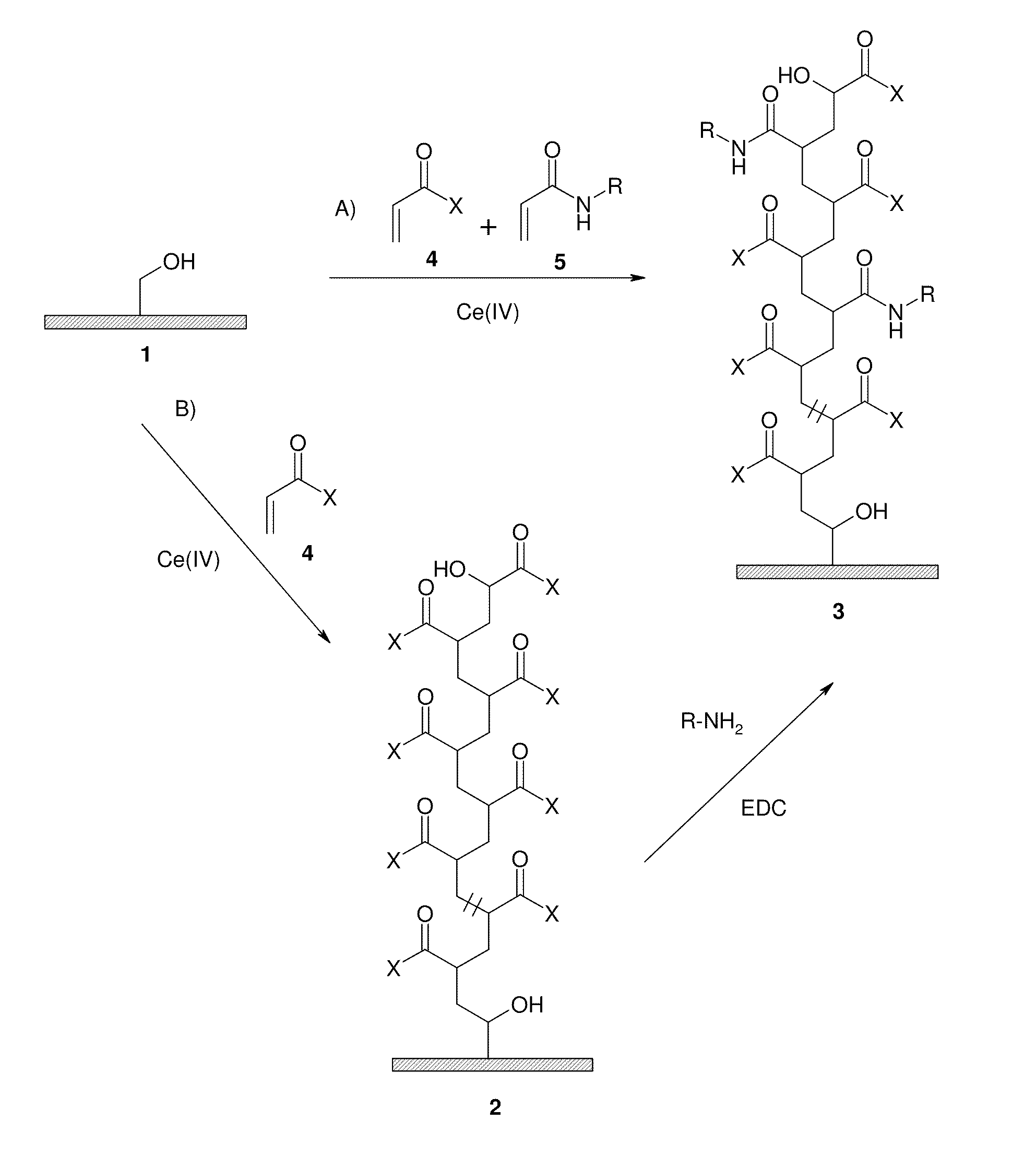
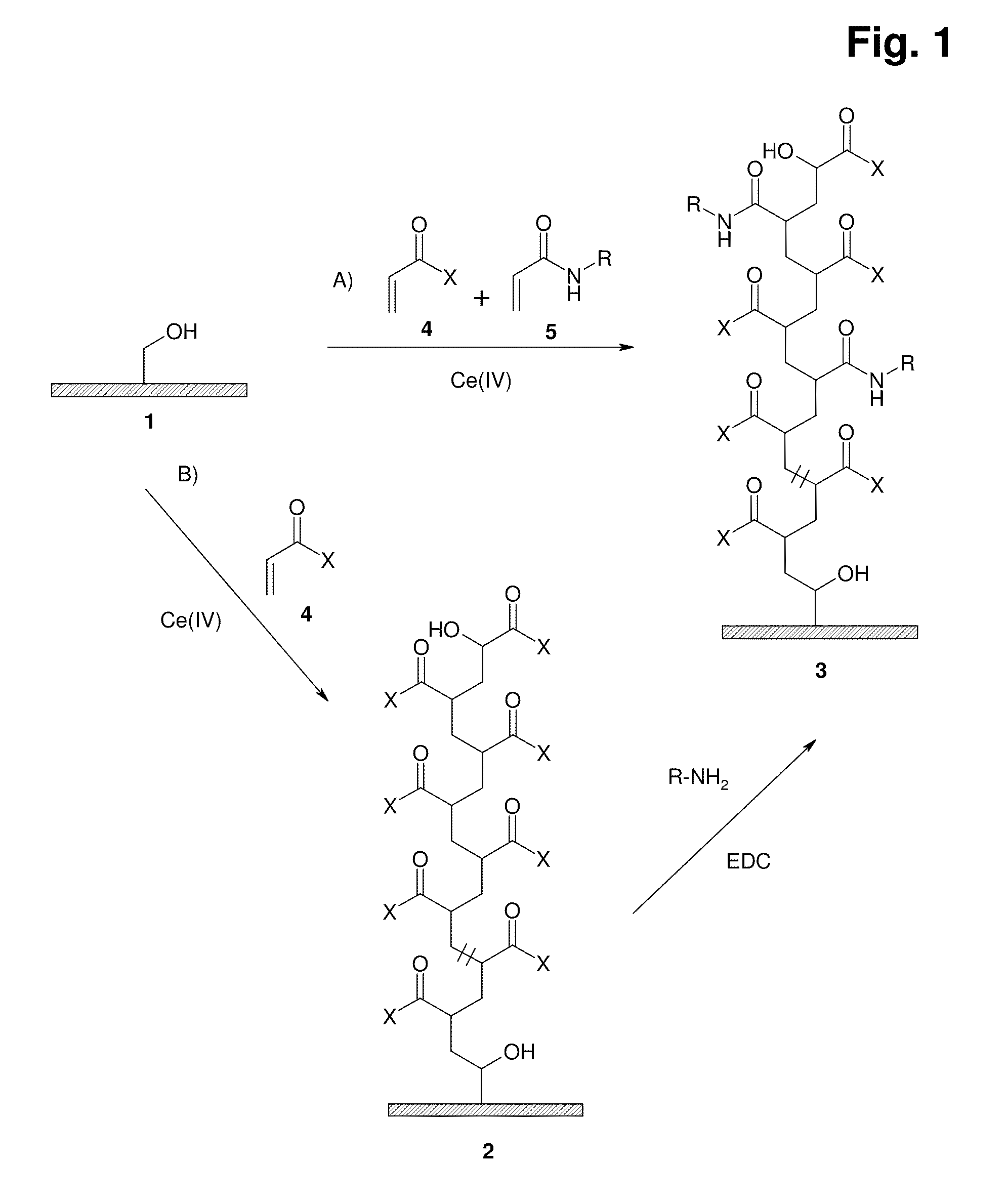
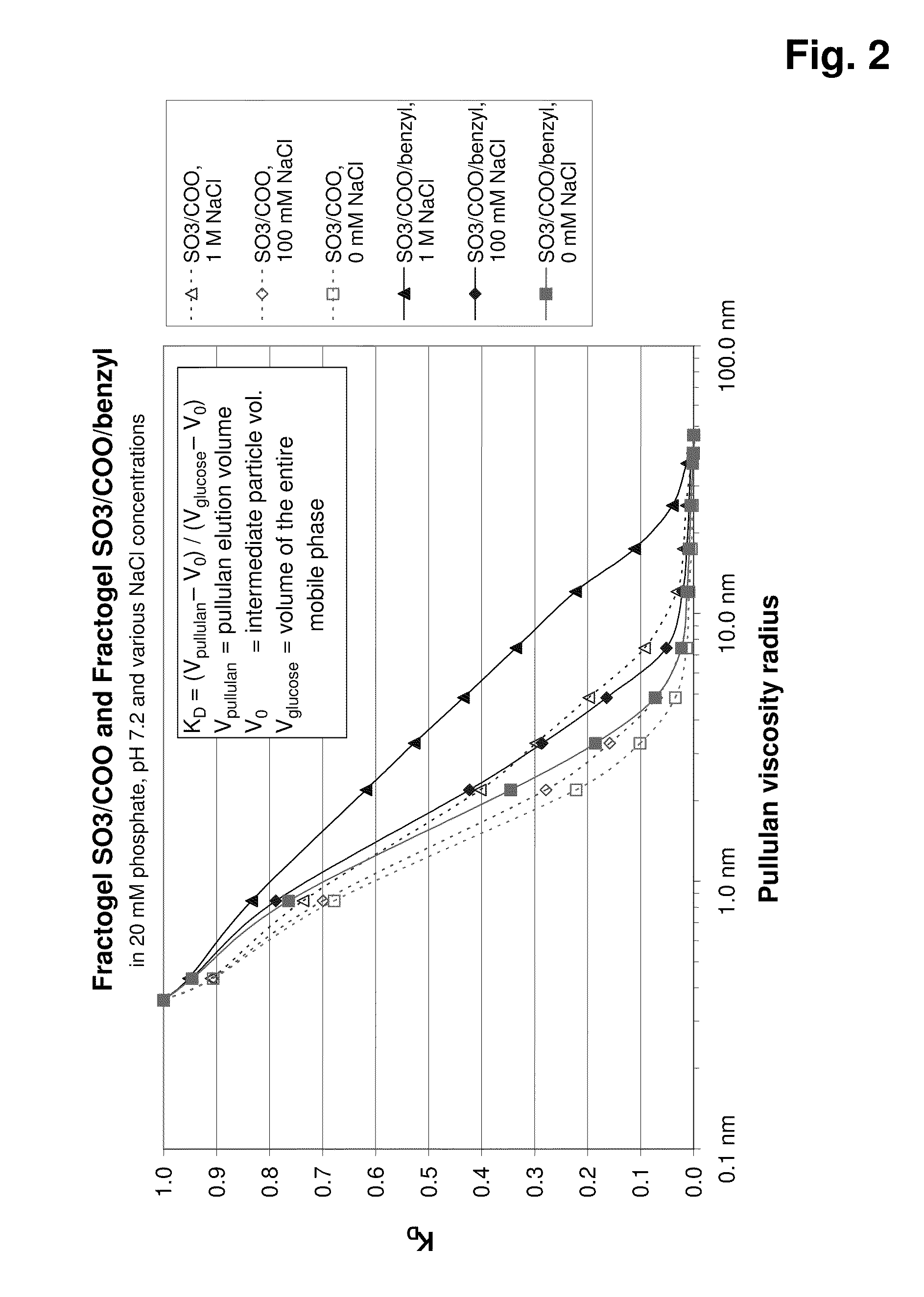
Graft copolymer for cation- exchange chromatography
a cation-exchange chromatography and copolymer technology, applied in the direction of cation-exchanger materials, separation processes, ion-exchangers, etc., can solve the problems of undeveloped special effects, undesired and undeveloped possibilities, and the use of sorbents to a limited exten
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Procedure for the Preparation of a Graft Copolymer from 2-acrylamido-2-methylpropanesulfonic Acid and Benzylacrylamide
Batch 05SW136
Procedure:
[0227]A suspension of 70 g of filter-moist Fractogel TSK HW65 (M) (washed with dilute mineral acid and deionised water), a solution of 32.3 g of benzylacrylamide in 250 ml of dioxane and a solution of 41.5 g of 2-acrylamido-2-methylpropanesulfonic acid and 25 g of 32% sodium hydroxide solution in 50 ml of deionised water is prepared in a glass reaction apparatus with a paddle stirrer. The suspension is made up to 475 ml with deionised water and adjusted to pH 4 using 32% sodium hydroxide solution or 65% nitric acid.
[0228]A starter solution comprising 13.7 g of ammonium cerium(IV) nitrate and 1.2 g of 65% nitric acid in 25 ml of deionised water is initially introduced in a dropping funnel with pressure equalisation. The entire apparatus is rendered inert by repeated (3×) evacuation and decompression with nitrogen. The suspension in the apparatus...
example 2
Procedure for the Preparation of a Graft Copolymer from Acrylic Acid and Benzylacrylamide
Batch 06SW297
Procedure:
[0231]A suspension of 77.9 g of filter-moist Fractogel TSK HW65 (M) (washed with dilute mineral acid and deionised water), a solution of 1.34 g of benzylacrylamide in 14.5 ml of dioxane and a solution of 18.0 g of acrylic acid in 50 ml of deionised water is prepared in a glass reaction apparatus with a paddle stirrer. The suspension is adjusted to pH 4 using 32% sodium hydroxide solution and made up to 375 ml with deionised water.
[0232]A further 6.72 g of benzylacrylamide are dissolved in 73 ml of dioxane in a dropping funnel with pressure equalisation and made up to 100 ml with deionised water.
[0233]A starter solution comprising 9.6 g of ammonium cerium(IV) nitrate and 1.2 g of 65% nitric acid in 25 ml of deionised water is initially introduced in a second dropping funnel with pressure equalisation. The entire apparatus is rendered inert by repeated (3×) evacuation and de...
example 3
Procedure for the Preparation of a Graft Copolymer from 2-acrylamido-2-methylpropanesulfonic Acid, Acrylic Acid and Benzylacrylamide
Batch 06SW085
Procedure:
[0237]A suspension of 69 g of filter-moist Fractogel TSK HW65 (M) (washed with dilute mineral acid and deionised water), a solution of 32.2 g of benzylacrylamide in 250 ml of dioxane, a solution of 25.9 g of 2-acrylamido-2-methylpropanesulfonic acid and 15.6 g of 32% sodium hydroxide solution in 50 ml of deionised water and 9.0 g of acrylic acid is prepared in a glass reaction apparatus with a paddle stirrer. The suspension is made up to 475 ml with deionised water and adjusted to pH 4 using 32% sodium hydroxide solution or 65% nitric acid.
[0238]A starter solution comprising 13.7 g of ammonium cerium(IV) nitrate and 1.2 g of 65% nitric acid in 25 ml of deionised water is initially introduced in a dropping funnel with pressure equalisation. The entire apparatus is rendered inert by repeated (3×) evacuation and decompression with ni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductivity | aaaaa | aaaaa |
| Substance count | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com