Compositions for radiolabeling diethylenetriaminepentaacetic acid (DTPA)-dextran
a technology of diethylenetriaminepentaacetic acid and radiolabeling technology, which is applied in the field of cancer, can solve the problems of decreased radiochemical purity and thermodynamic stability, and achieve the effects of preventing oxidative degradation, high radiochemical purity, and enhanced radiolabeling efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
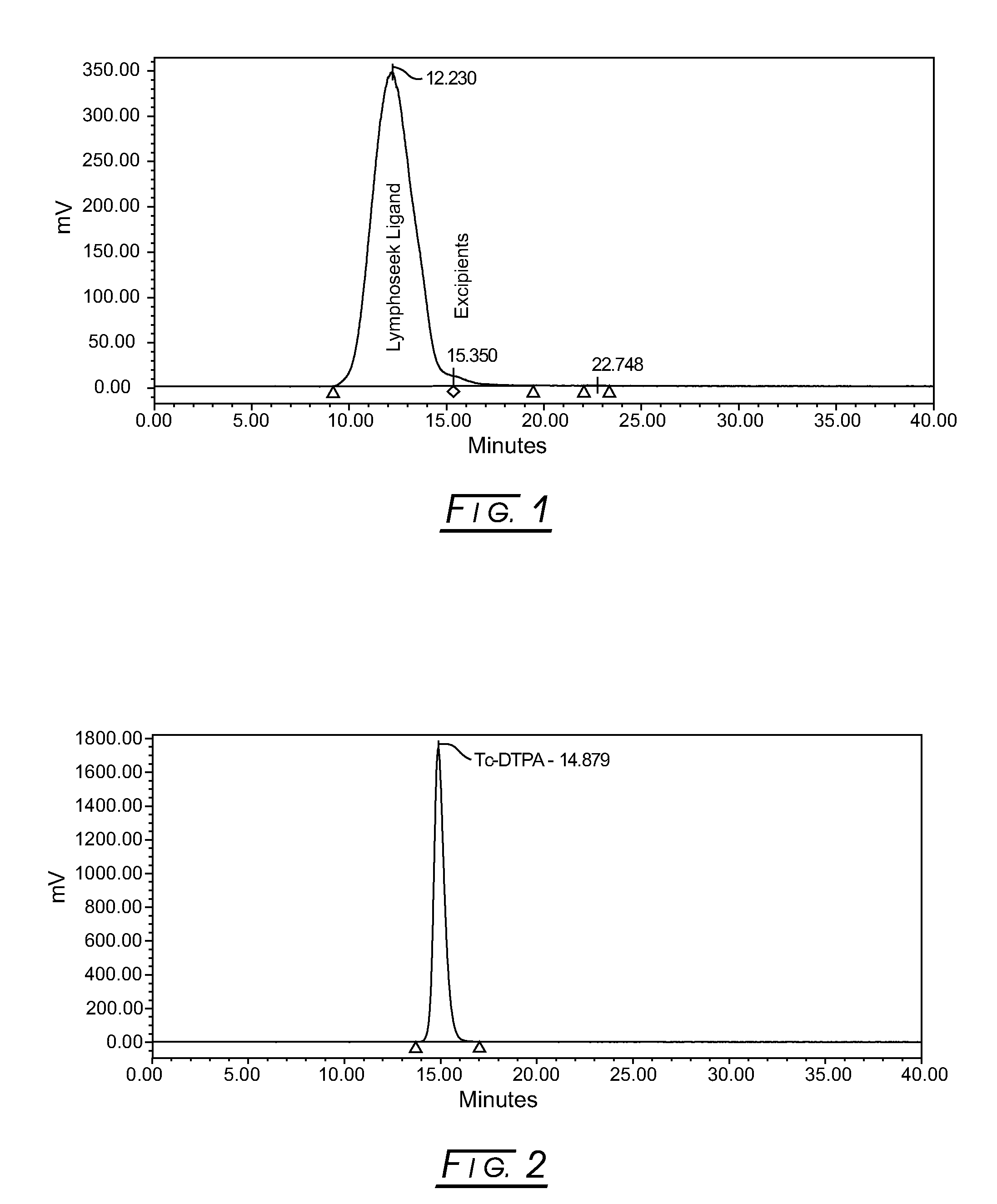
Elution Profiles of 99mTc-Labeled DTPA-Mannosyl-Dextran and DTPA
[0035]FIG. 1 shows a typical elution profile for reconstituted 99mTechnetium-labeled Lymphoseek Ligand Drug Product (99mTc-DTPA-mannosyl-dextran), Lot NMK001, measured by a radioactivity (Nal, set at 1000 cps / Volt) detector using Size Exclusion Chromatography (SEC). The conditions for this SEC radiochemical purity method are as follows: a TSKgel column, Tosoh Bioscience, G3000PWXL (7.8×30 cm, 6 μm, with a column temperature of 25±5° C.) is employed with an isocratic mobile phase of 50 mM phosphate buffer, pH 7.2, and 300 mM sodium chloride. The lyophilized vial is reconstituted with 0.8 cc of 10 milliCuries of 99mTc-pertechnetate, mixed, and allowed to radiolabel for at least 10 minutes at ambient room temperature prior to partially neutralizing the sample in 0.2 cc Phosphate-buffered saline. A refrigerated drug product sample, 15 μL, is injected and run at 0.6 mL / minute for a run time of 40 minutes; the retention time ...
example 2
Initial Pilot Formulation: Investigating Interfering Excipients
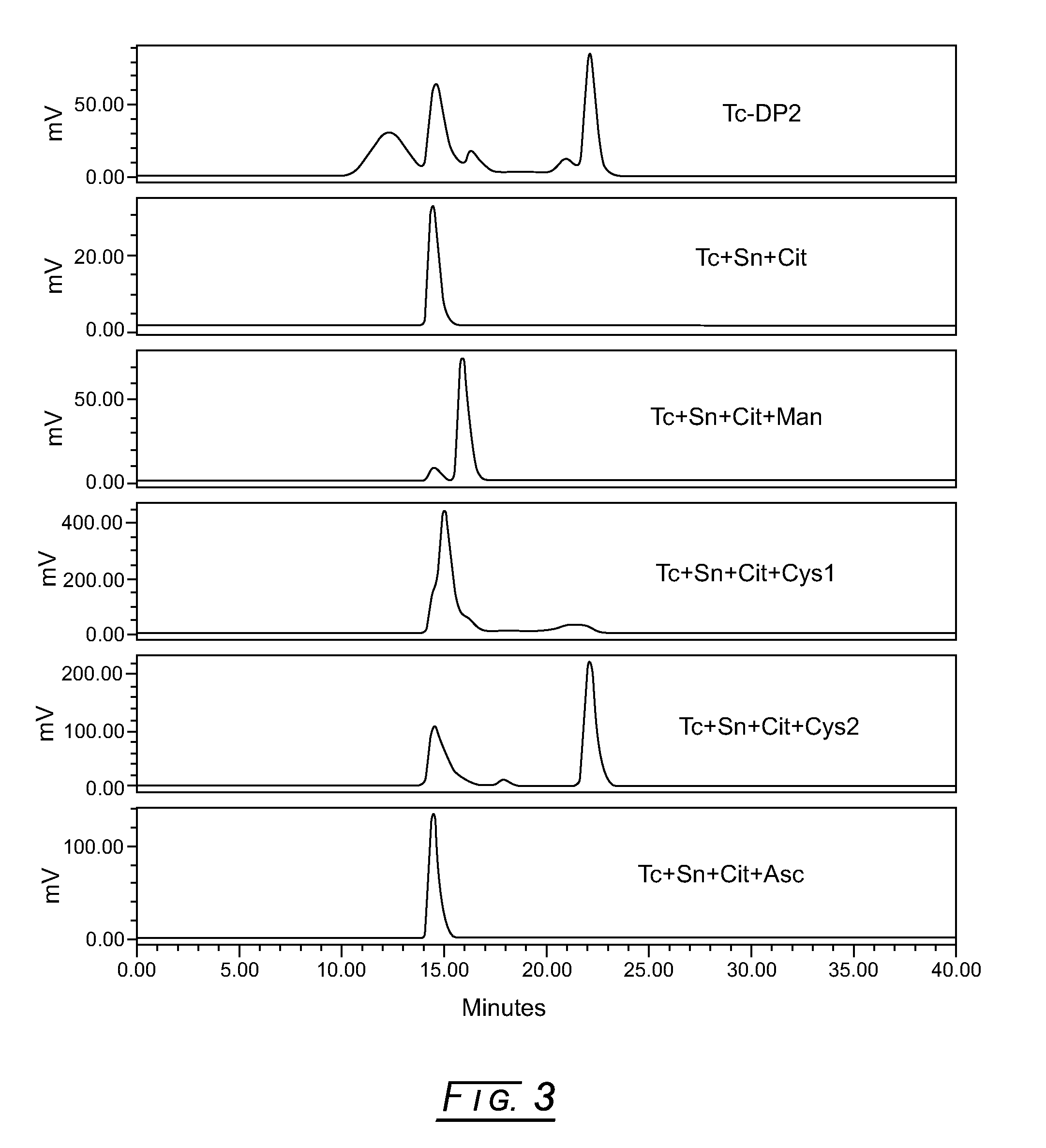
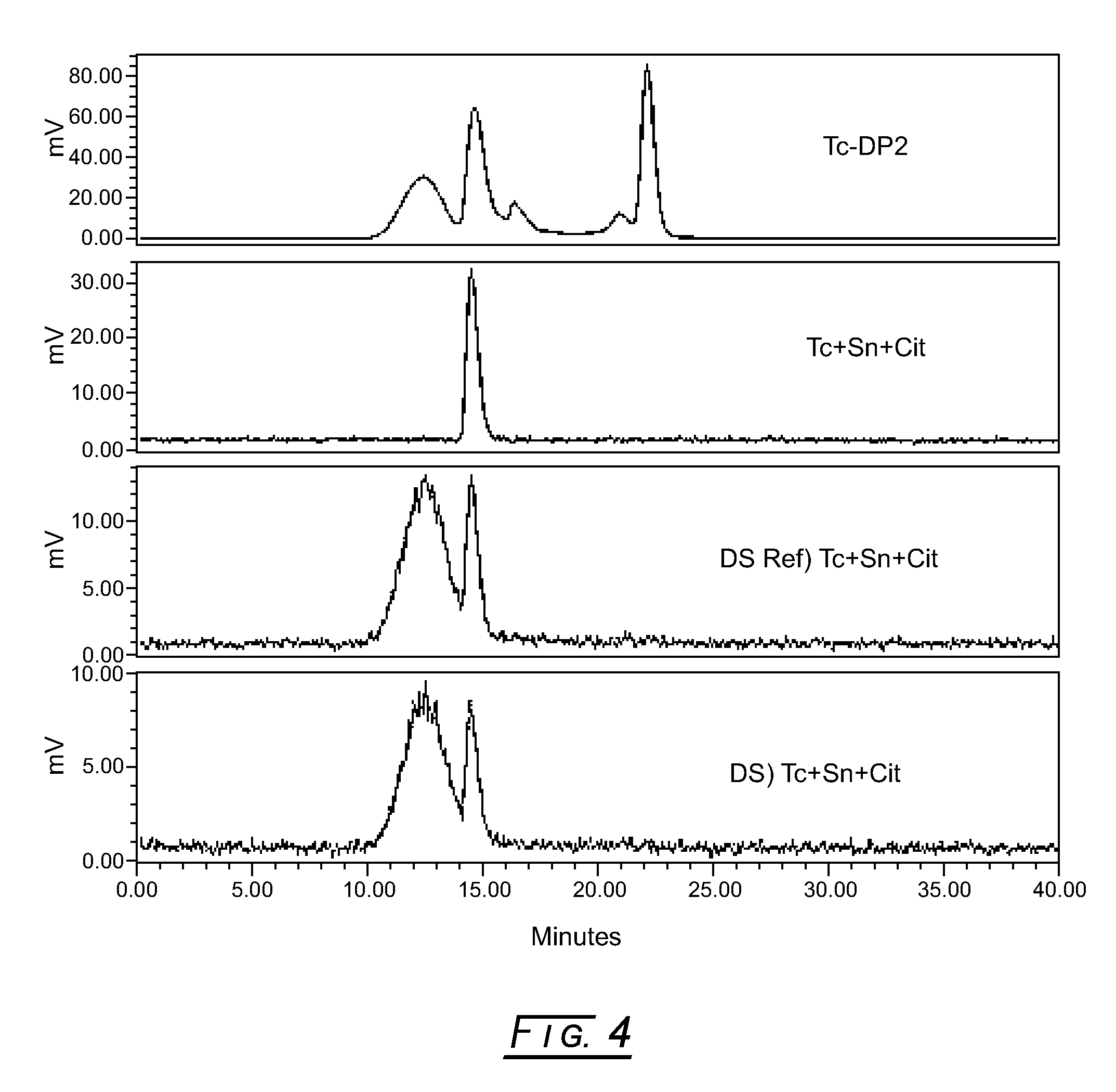
[0038]In FIG. 3, the topmost stacked radiochemical elution profile shows the initial lyophilized formulation pilot (5 μM (0.1 mg / mL) DTPA-mannosyl-dextran, 20 mM Sodium Citrate, pH 5.6, 5.7 mM Sodium L-Cysteine, 2% (w / v) D-Mannitol and 75 μg / mL Stannous Chloride, Dihydrate) reconstituted with 10 milliCuries 99mTc-pertechnetate and run via the SEC radiochemical purity method. (The initial lyophilized drug product formulation pilot just preceded the development of the SEC radiochemical purity method.) This elution profile clearly shows that the 99mTc-DMD peak has less than about 25% radiochemical purity.
[0039]The following screening method (in the order of addition) was employed to determine potential interfering excipients in pilot formulations: (1) for drug substance placebo formulations, add 50 μL degassed saline to a 1.5 mL plastic test tube with a cap; for drug substance formulations, add 50 μL of 1.2 mg / mL DTPA-manno...
example 3
Screening for pH Buffers, Transchelator and Bulking Excipients for Enhanced Radiolabeling of DTPA-Mannosyl-Dextran
[0042]FIG. 5 is a stacked radiochemical elution profile for liquid DTPA-mannosyl-dextran drug substance placebo formulation pilots containing a Sodium Phosphate pH buffer and different combinations of transchelator, reducing agents and bulking agents, as measured by the SEC radiochemical purity method. The topmost stacked radiochemical elution profile shows a small 99mTc-labeled interference peak with the 20 mM Sodium Phosphate buffer at pH 4 and 1.5 mg / mL Sodium Ascorbate. The second through the sixth elution profiles shows 20 mM Sodium Phosphate, pH 4, 75 μg / mL SnCL2.2H2O and 12.5 mCi 99mTc-pertechnetate with the following respective potential excipients: 1 mg / mL Sodium Citrate; 1% PEG 8000; 1 mg / mL Sodium Citrate and 1.5 mg / mL Sodium Ascorbate; 1.5 mg / mL Sodium Ascorbate and 1% PEG 8000; and 1.5 mg / mL Sodium Ascorbate, 1 mg / mL Sodium Citrate and 1% PEG 8000. They all ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| average molecular weight | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com