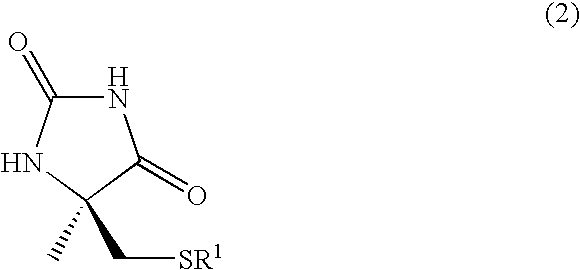
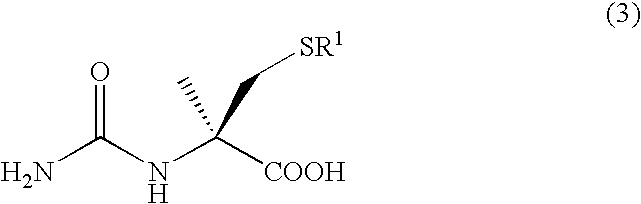
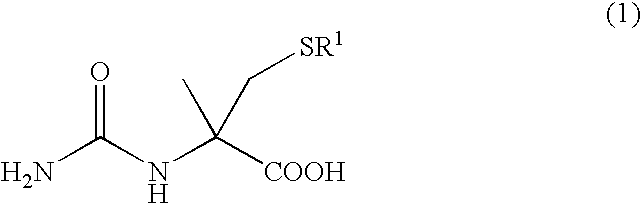
Process for producing optically active alpha-methylcysteine derivative
a technology of lor dmethylcysteine and alpha-methylcysteine, which is applied in the field of process for producing optically active lor dmethylcysteine derivatives, can solve the problems of complex process 5 process, inability to easily stably secure industrial scale, and various kinds of expensive reagents, and achieves high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
Method for producing racemic 5-methyl-5-tert-butylthiomethylhydantoin
[0183]In a reactor provided with a nitrogen balloon, a 5 wt % aqueous sodium hydroxide solution (9.6 g, 12 mmol) and tert-butyl mercaptan (1.13 mL, 10 mmol) were mixed at 0° C., and the mixture was stirred for 10 minutes. Then, chloroacetone (0.79 mL, 10 mmol) was added to the mixture, and reaction was performed at room temperature for 2 hours. The reaction solution was light yellow and separated into two phases, A Dimroth condenser was attached to the reactor, and NaCN (588 mg, 12 mmol), (NH4)HCO3 (2.77 g, 35 mmol), and 28% ammonia water (3.1 mL) were added to the reactor to prepare a homogeneous solution. Then, the temperature was increased to 55° C. to 60° C. After stirring under heating for 6 hours, the solution was cooled to 0° C., and conc. hydrochloric acid was added to the reaction solution to control the pH to 7.0 to 7.6. The resulting white crystals were filtered off and analyzed by 1H NMR. As a result, i...
reference example 2
Method for producing 5-(2-methoxyphenylmethyl)-5-methyl-hydantoin
[0184]First, 2-methoxyphenylacetone (16.4 g, 100 mmol) was mixed with 164 g or water, and NaCN (5.88 g, 120 mmol), (NH4)HCO3 (27.7 g, 350 mmol), and 27.7 g of 28% ammonia water were added to the resulting mixture. After stirring at 50° C. for 4 hours and at 60° C. for 12 hours, the mixture was allowed to cool down to 23° C., and then controlled to pH 7.5 by adding conc. hydrochloric acid. The precipitated solid was filtered off, washed with toluene, and dried under reduced pressure to obtain 22.10 g (yield 94.5%) of the title compound.
[0185]1H NMR (300 MHz, CDCl3) δ: 7.10-6.88 (m, 4H), 5.49 (brs, 1H), 3.86 (s, 3H), 3.20 (d, 1H), 2.97 (d, 1H), 1.49 (s, 3H)
reference example 3
Method for producing racemic N-carbamoyl-5-tert-butyl-α-methylcysteine
[0186]Racemic 5-methyl-5-thiomethylhydantoin (4.77 g, 22.1 mmol) was dissolved in a 10% aqueous sodium hydroxide solution (75 g), and the resultant solution was refluxed for 72 hours. After being allowed to cool down to room temperature, the reaction solution was sampled for confirming the production of racemic S-tert-butyl-α-methylcysteine by HPLC (column: COSMOSIL AR-II (produced by Nacalai Tesque Inc.), mobile phase: potassium dihydrogen phosphate-aqueous phosphate solution (pH 2.0) / acetonitrile=97 / 3, flow rate: 1.0 ml / min, detection wavelength: 210 nm, column temperature: 40° C., retention time: 21.15 min). After the reaction solution was adjusted to pH 8 with conc. hydrochloric acid, the solution was heated to 70° C., and a solution of potassium cyanate (2.07 g) in distilled water (10 mL) was added dropwise to the solution over 20 minutes. After the completion of the addition, the resultant mixture was stirre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| L- or D-optically active | aaaaa | aaaaa |
| optically active | aaaaa | aaaaa |
| chiral HPLC | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com