Polypeptides and polynucleotides encoding same and use thereof in the treatment of medical conditions associated with ischemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
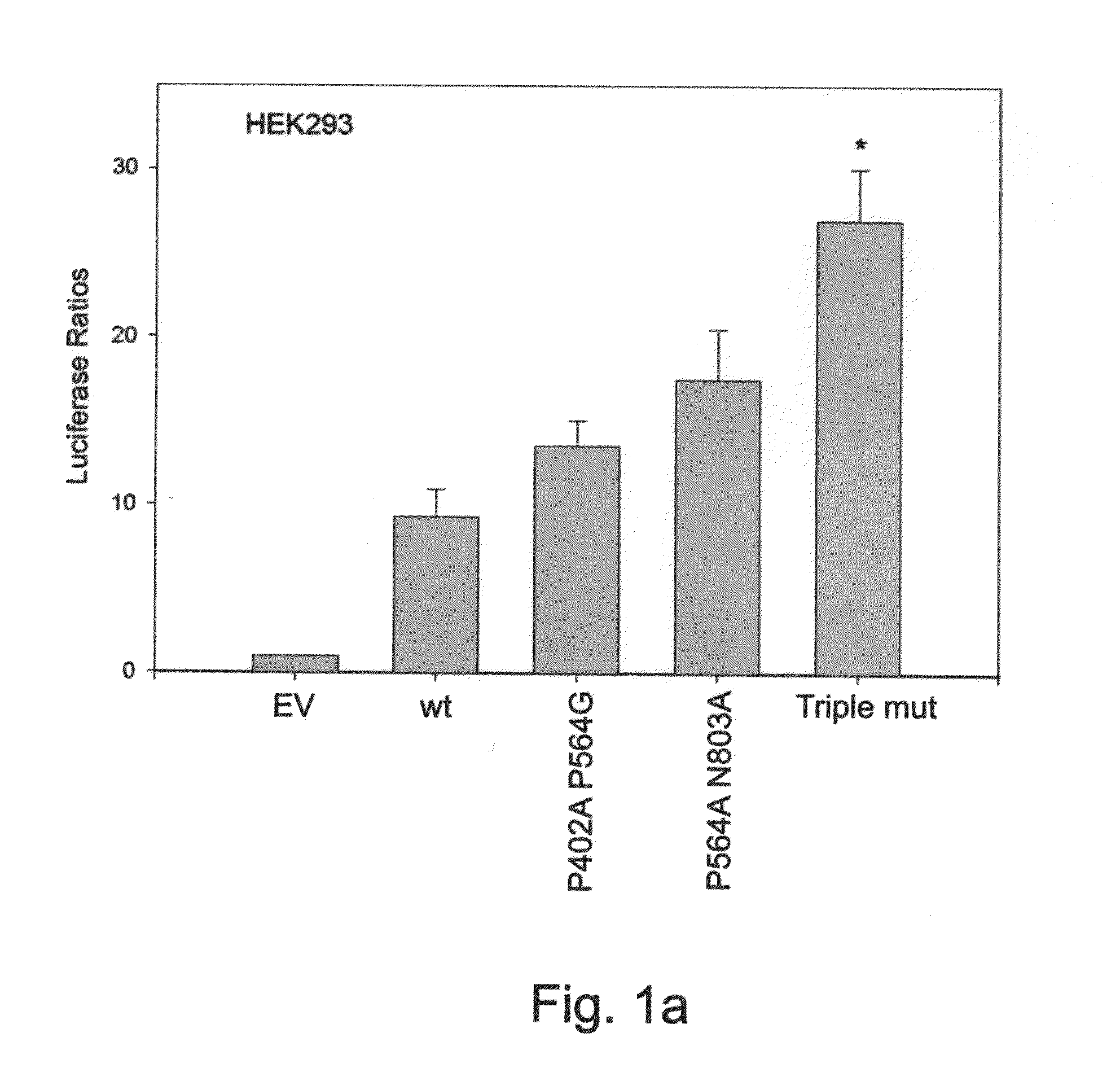
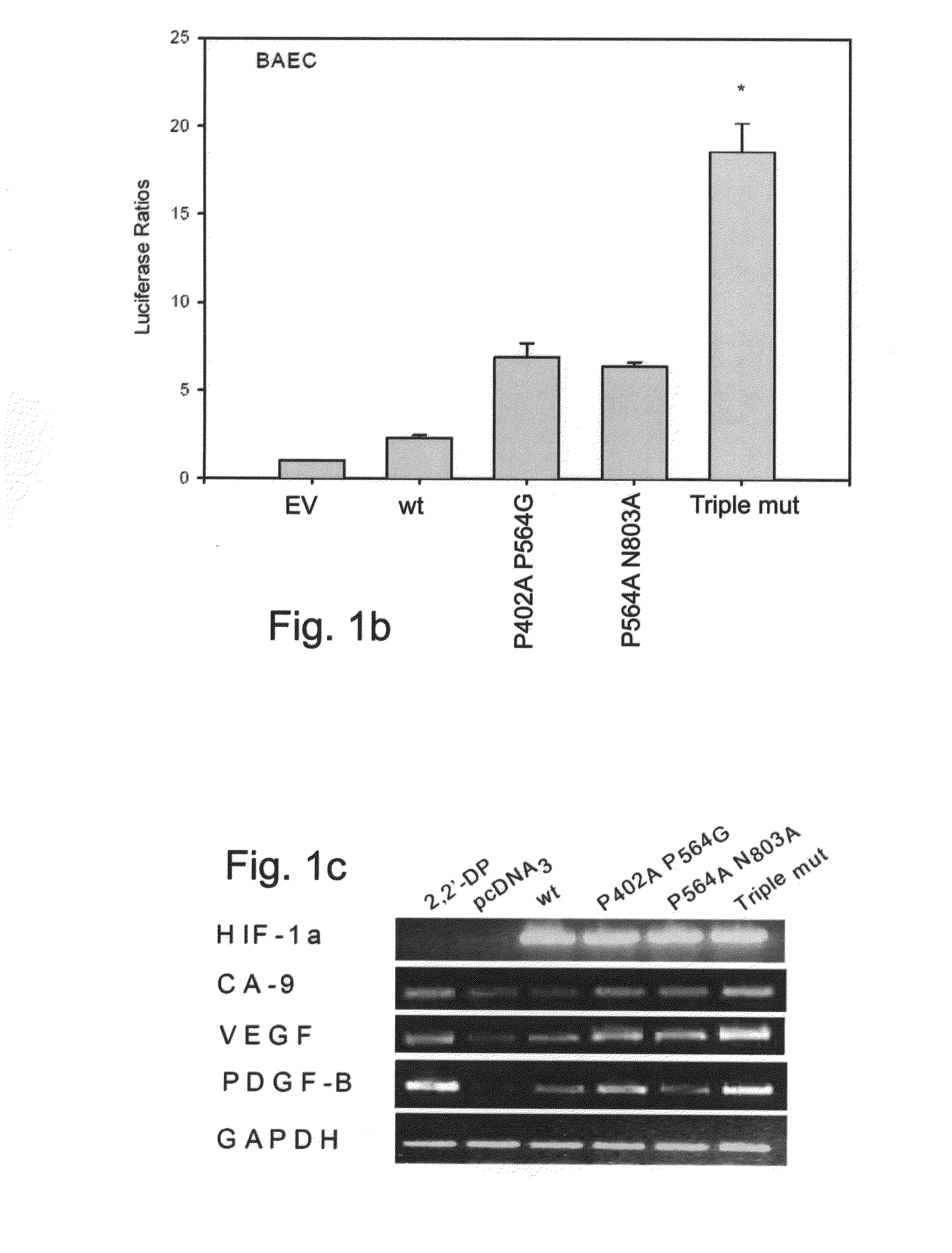
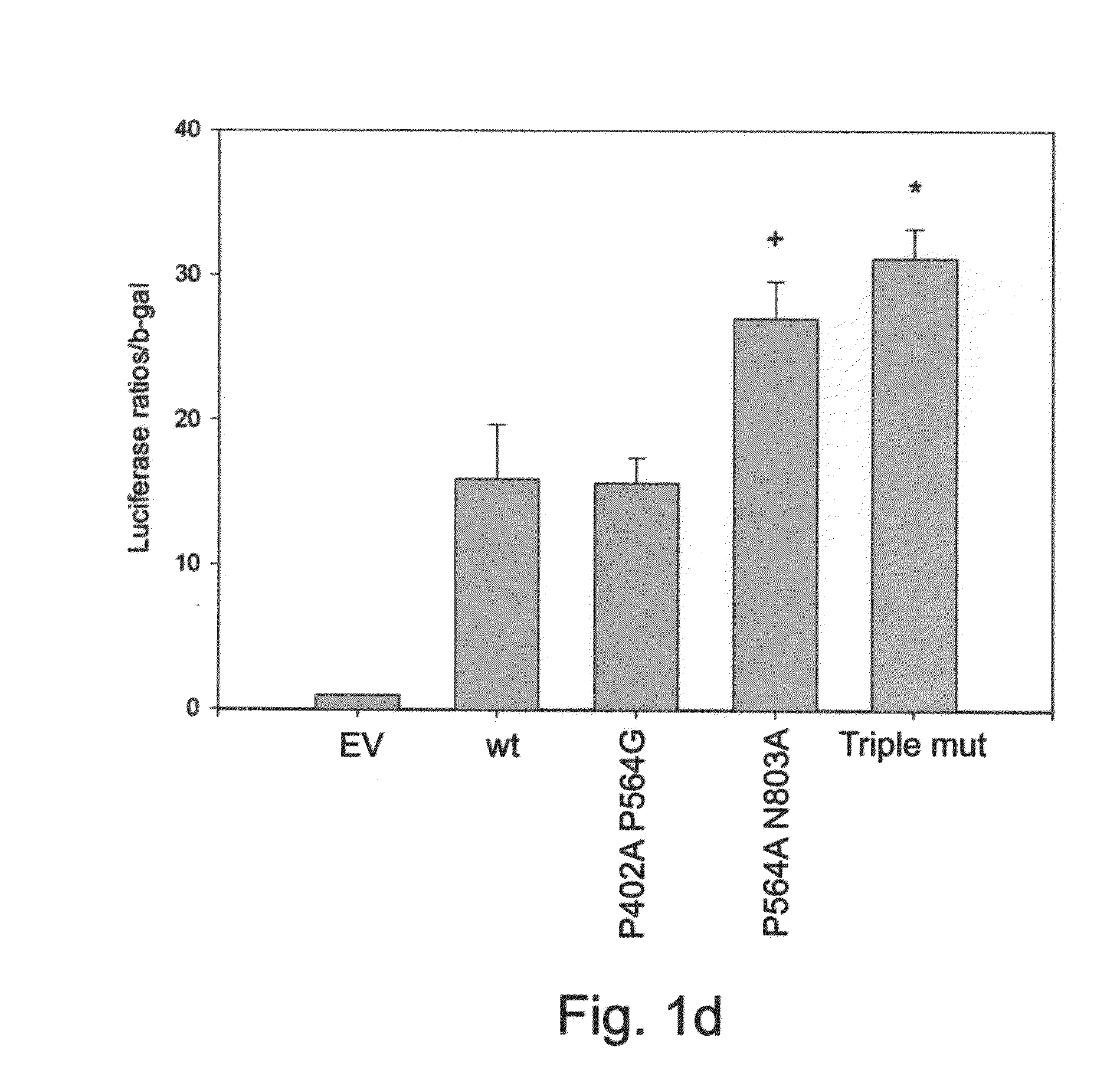
Triple Mutant HIF-1α Shows Greater Transcriptional Activity than P402A P564G and P564A N803A Double Mutants
[0177]Point mutations P402A and P564G within HIF-1α were previously shown to abrogate its interaction with the tumor suppressor VHL, thus preventing its subsequent proteosomal degradation. This resulted in stabilized HIF-1α during normoxia, reaching activity levels comparable to hypoxic condition. Similarly, HIF-1α with point mutations P564A N803A was demonstrated to be as active in normoxia as wild-type HIF-1α following treatment with the hypoxia mimetic iron chelator 2,2′-dipyridyl. The present inventors hypothesized that a combination of all three mutations may result in a mutated HIF-1α, termed ‘Triple mutant’, which is more active than the P402A P564G mutant and more stable than the P564A N803A mutant, thus making it more potent for therapeutic angiogenesis purposes.
[0178]Materials and Methods
[0179]Cell culture: The following cell lines were used: Bovine aortic endothelial...
example 2
Activation of C-Transactivation Domain is Essential for Optimal HIF-1α-Mediated Angiogenesis
[0189]In order to assess the significance of the triple mutant's increased transcription ability in terms of therapeutic angiogenesis, a comparison of the different mutants was carried out using an in-vitro angiogenesis assay following transfection of the HIF-1α constructs in human umbilical vein endothelial cells (HUVEC).
[0190]Materials and Methods
[0191]In-vitro angiogenesis assay: Transfected or infected HUVECs, grown in 60 mm dishes, were maintained for 24 hours in EGM-2 medium followed by medium replacement with EBM-2. Cells were maintained in EBM-2 for further 24 hours, prior to assay. Cells were then trypsinized and seeded at concentration of 50,000 cells per well on 24-well plate pre-coated with growth factor reduced matrigel (BD Biosciences, USA). Capillary and tube formation was tracked for 8 hours. Quantitation of capillary formation was performed by counting the number of capillary...
example 3
Expression and Specificity of the PPE-1-3x Promoter in Ischemia
[0196]A prerequisite for the feasibility and safety of systemic pro-angiogenic gene therapy is the sufficiently robust and specific expression within the target ischemic tissue. In order to characterize the expression and specificity of a modified PPE-1 promoter in the setting of ischemia, an adenovirus expressing GFP under the regulation of PPE-1-3 x was used (Ad-PPE-1-3x-GFP).
[0197]Materials and Methods
[0198]Construction of adenoviral vectors: Ad-PPE-Triple, Ad-CMV-Triple, and Ad-CMV-wt vectors were generated by homologous recombination by Vector Biolabs (Philadelphia, USA). Successful creation of viral constructs was verified by PCR, following which two rounds of plaque purification were performed. Viral plaques subsequently underwent large-scale amplification in HEK293, followed by CsCl purification.
[0199]Animals: Twelve-week old female C57BL / 6J mice (Harlan Laboratories Ltd., Jerusalem, Israel) were used. All animal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com