Biodegradable bioactive agent releasing matrices with particulates
a bioactive agent and bioactive agent technology, applied in the field of biodegradable polymeric matrices, can solve the problems of achieve high bioactive agent loading, high hydrophilic bioactive load, and good adhesion to the device surfa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Controlled Delivery of Nonspecific Fab from Poly(Lactide-Co-Caprolactone) Microparticulate Coatings
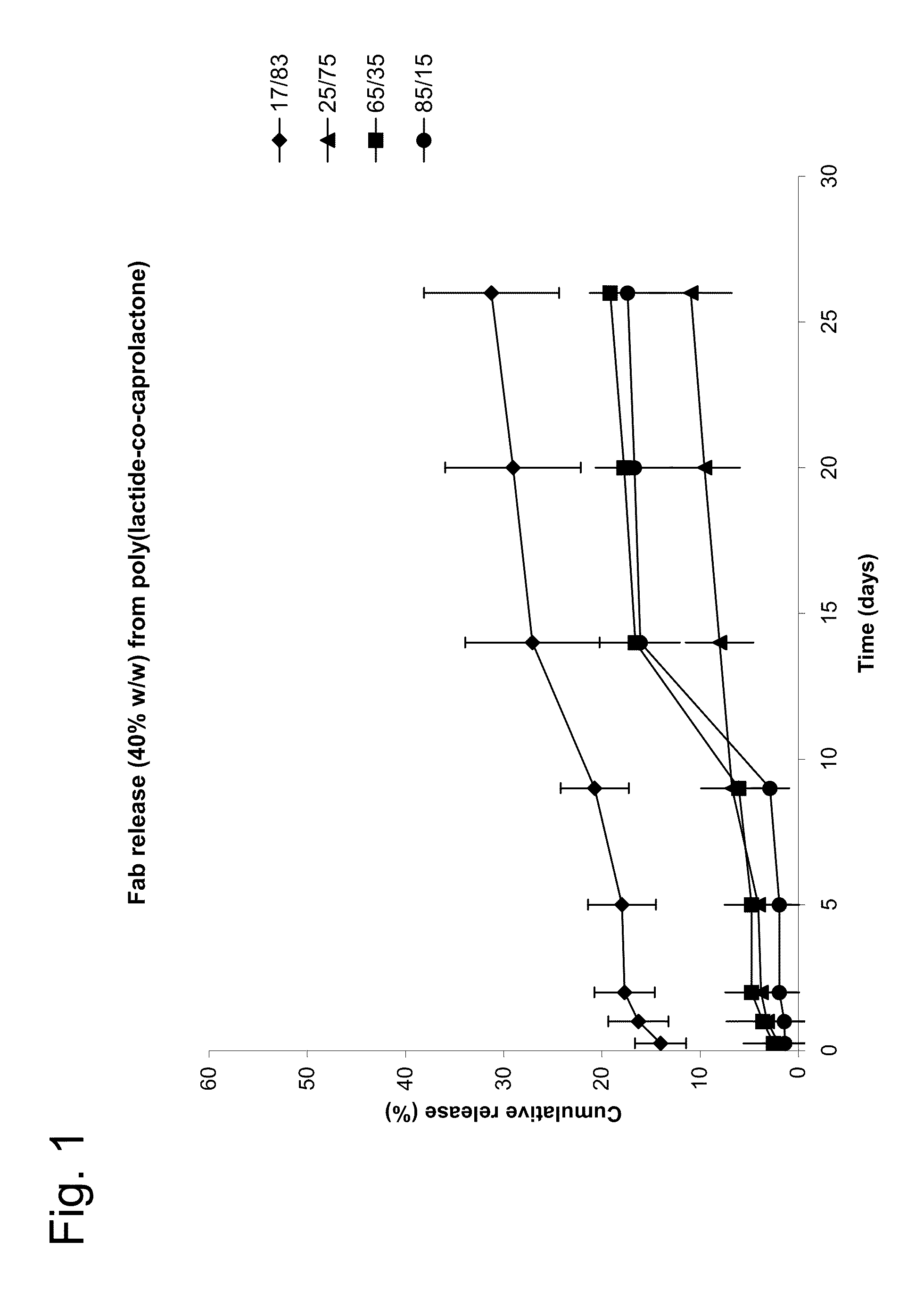
[0135]The controlled release characteristics and capacity of coatings formed from various poly(lactide-co-caprolactone) copolymers were investigated using high protein loadings (˜40% w / w).
[0136]Helical intravitreal coil implants constructed from MP-35 alloy (see commonly assigned U.S. Pub. No. 2005 / 0019371) were used as the medical device on which the coatings were formed.
[0137]Poly(lactide-co-caprolactone; pDLCL), prepared using various DL:CL ratios, was synthesized by Lakeshore Biomaterials (Birmingham, Ala. 35211). The pDLCL polymers used were pDLCL17 / 83 8E (IV=0.73), pDLCL 25 / 75 8E (IV=0.75), pDLCL 65 / 35 4A (IV=0.43) and pDLCL 85 / 15 (8E IV-0.81).
[0138]Nonspecific Fab spray-dried particles containing 70% nonspecific Fab, 30% trehalose, and 0.1% Tween-80™ were obtained from SurModics Pharmaceuticals (Birmingham, Ala. 35211).
[0139]Coating compositions were prepared by dispersing 20 mg...
example 2
Controlled Delivery of Nonspecific Fab from Poly(Lactide-Co-Caprolactone) / PEG1000-45PBT-55 Microparticulate Coatings
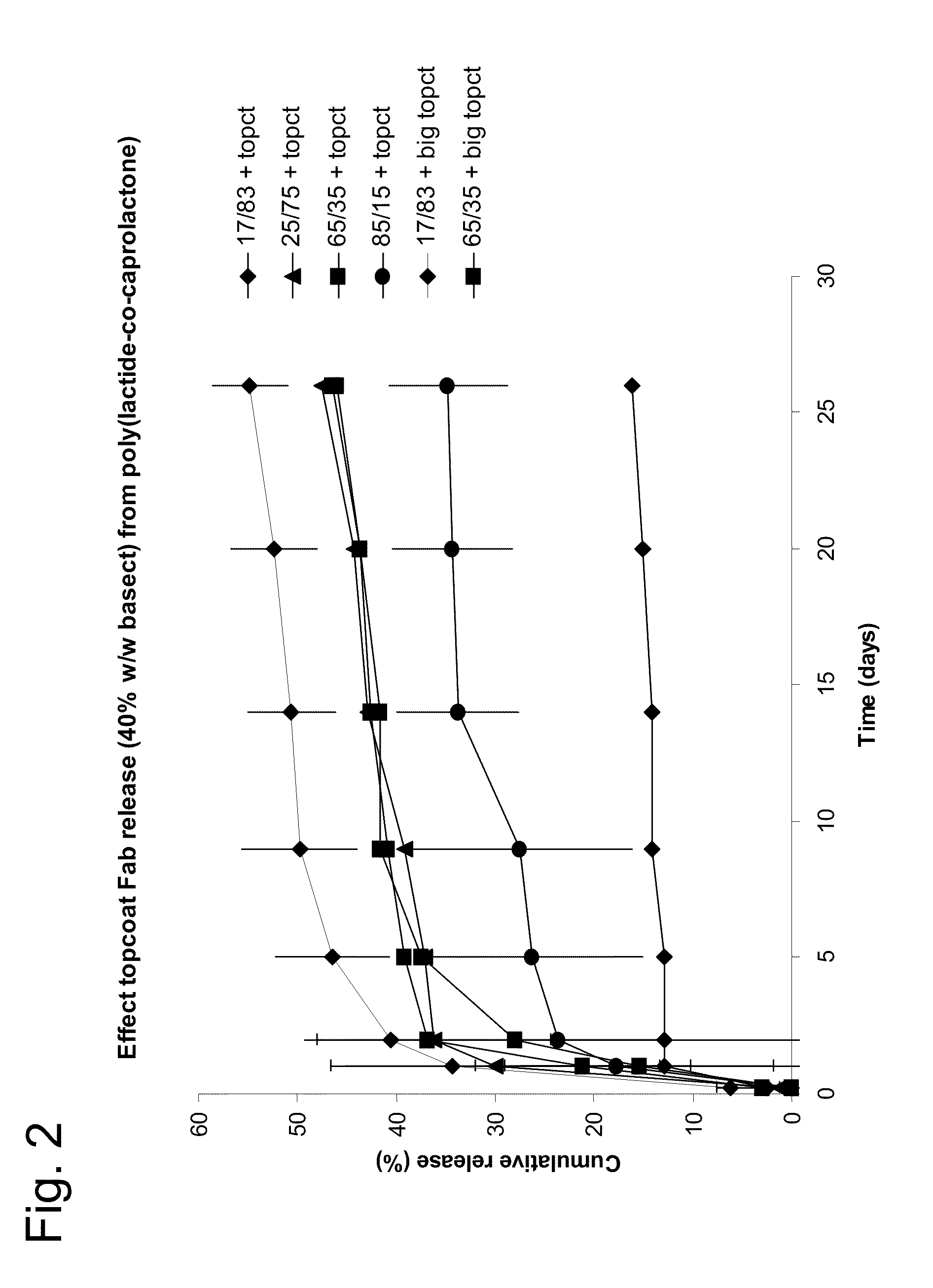
[0145]The controlled release characteristics and capacity of coatings formed from various poly(lactide-co-caprolactone) copolymers along with a biodegradable poly(butyleneterephthalate-co-ethylene glycol) copolymer were investigated using high protein loadings (˜40% w / w).
[0146]Helical intravitreal coil implants, pDLCL copolymers, and nonspecific Fab spray-dried particles, as described in Example 1, were used to prepare the coatings. The polymer PEG1000-45PBT-55 is a copolymer of poly(butyleneterephthalate-co-ethylene glycol) copolymer with 45 wt. % polyethylene glycol having an average molecular weight of 1000 kD and 55 wt. % butyleneterephthalate. PEG1000-45PBT-55 is commercially available from OctoPlus (Leiden, Netherlands) under the product name PolyActive™.
[0147]Coating compositions were prepared by dispersing 20 mg of Fab protein particles in 5 mL of chloroform co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| average diameter | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com