Modified Molecular Arrays
a molecular array and molecular technology, applied in the field of modified molecular arrays, can solve the problems of unsuitable for modifying the surface of another support, non-specific binding of nucleotides to the array, and difficult preparation of arrays at the single molecule level than at the multi-molecule level, and achieve the effect of less reactiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Polyacrylamide-Based Surface
Application of the Polyacrylamide Hydrogel to the Glass Slides
[0221]Method I—Two slides (optionally silanised) were assembled with a silicon gasket in between to form a polymerisation cell. The slides and the gasket were held together with binder clips. Polymerisation mixture (800 μl) was injected in each polymerisation cell. The polymerisation proceeded at room temperature for 1.5 hr. The polymerisation cells were then disassembled and the slides washed thoroughly under running MilliQ H2O. The slides were then dried and stored under argon.
[0222]Method II—Slides (optionally silanised) were put into a clean coplin jar. The polymerisation mixture was poured into the jar to cover the slides. The polymerisation proceeded at room temperature for 1.5 hr. The slides were then removed from the coplin jar one by one and rinsed under running MilliQ H2O. The slides were then introduced in clean plastic vials containing MilliQ H2O and vortexed for 20 sec...
example 2
Immobilisation of Polynucleotides on Polyacrylamide Surfaces
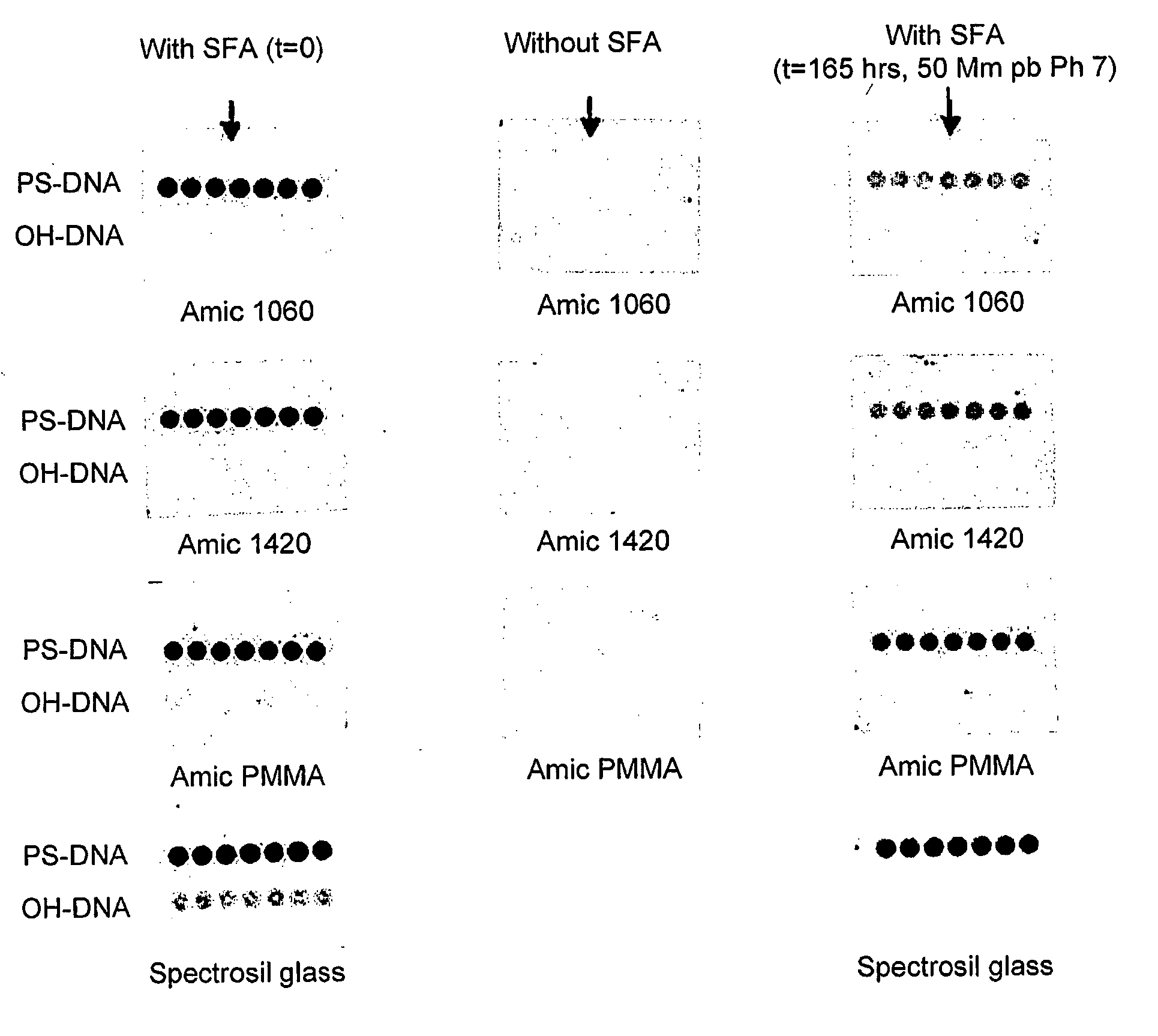
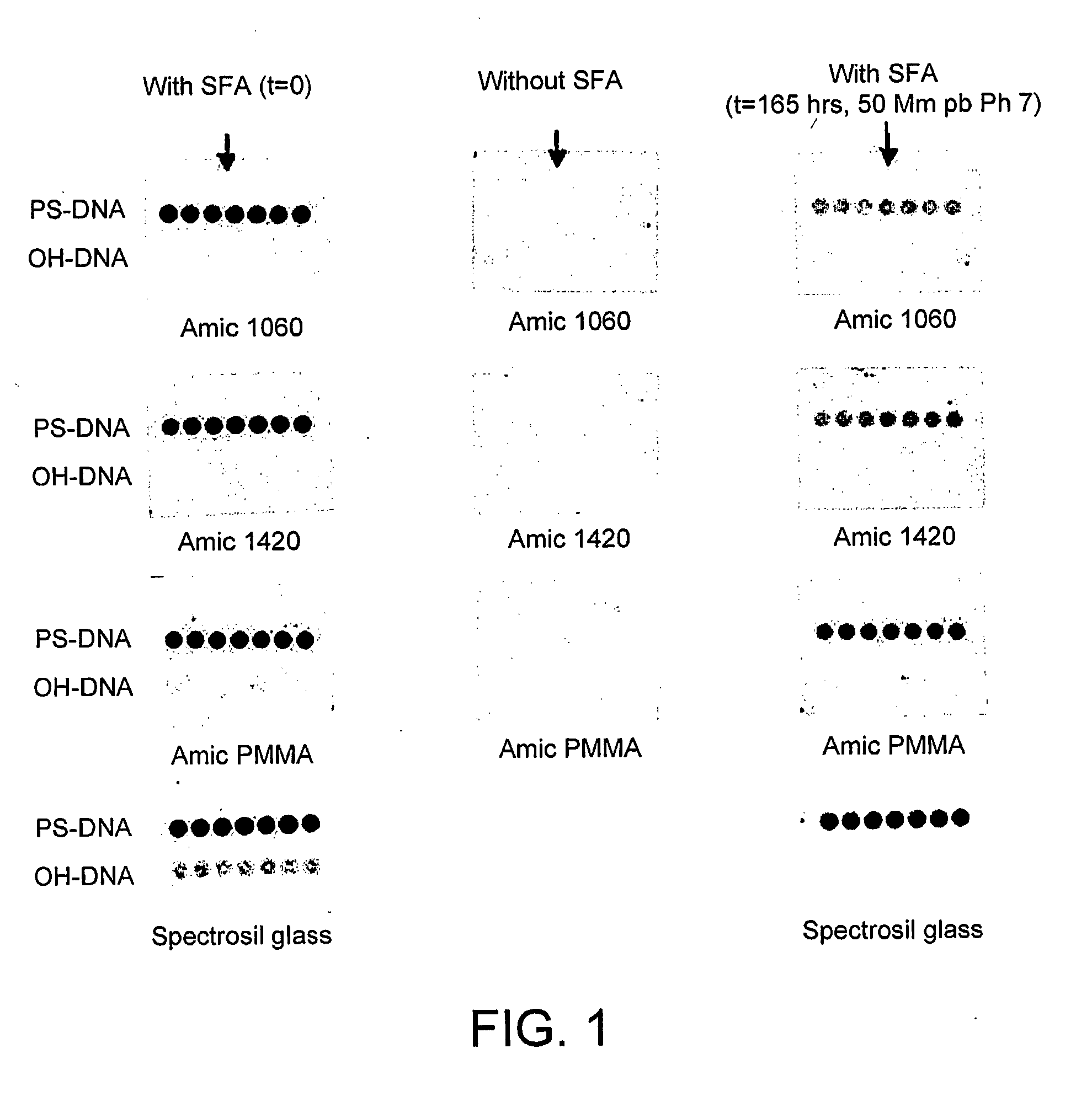
[0223]The following constitutes representative procedures for the immobilisation of 5′-phosphorothioate-modified polynucleotides to the new surface. Oligos with a 3′-fluorescent label are typically used for appraising fundamental surface characteristics.
A: Bulk Application (Suitable for Microarrays)
[0224]Polynucleotide (1 μM) in the printing buffer (potassium phosphate 100 mM, pH 7) was applied to the surface as 1 μl drops. The slide was then placed in a humid chamber at room temperature for 1 hour. The printed slide was then rinsed with MilliQ H2O and vortexed in hot washing buffer (Tris HCl 10 mM, EDTA 10 mM, pH 8 (at 80-90° C.)). The printed slide was finally rinsed with MilliQ H2O, dried under a flow of argon and stored in the dark before imaging.
[0225]To immobilise polynucleotides over a large area, a gasket was placed onto the slide, the polynucleotide solution injected into the chamber formed and a cover slip placed ...
example 3
Thermal Stability of the Immobilised Polynucleotides
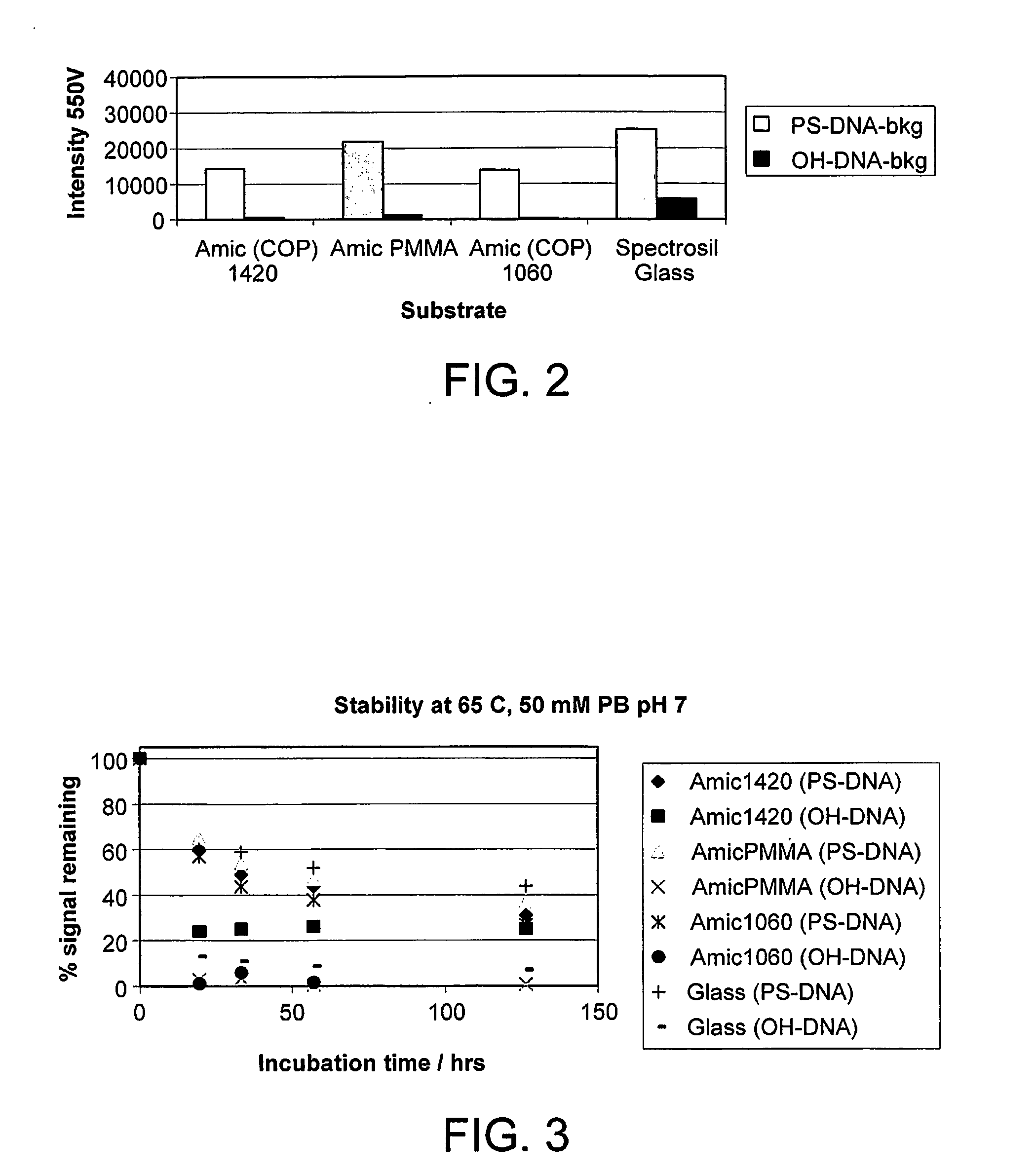
[0227]A: Bulk application (suitable for microarrays)—A printed slide was imaged with a fluorescence scanner in the presence of a fluorescence reference control slide containing attached Cy3 dye. The printed slide was then incubated in a jar containing printing buffer at a preset temperature in the dark. The slide was imaged at regular intervals and the fluorescence intensity recorded. The fluorescence intensity of a spot is proportional to the amount of polynucleotide immobilised on that area. A plot of the variation of the fluorescence intensity with time gave a stability profile for attached polynucleotide on the polyacrylamide surface.
[0228]B: Single molecule array application—A slide was printed in a flow cell as described above. Printing buffer is injected through the cell at a rate of 1 ml / min, at a preset temperature, and the slide imaged at regular intervals using a custom-made total internal reflection fluorimeter instrume...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| areas | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com