Raman-active reagents and the use thereof
a technology of raman and active reagents, applied in the field of ramanactive reagents, can solve the problems of limited reliable individual and quantitative detection of multiple analytes in a single sample, limited use of fluorescence spectroscopy, and insufficient sensitivity of conventional raman spectroscopy for use as immunoassay readout methods, etc., to achieve the effect of reducing labor costs, signal stability, and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
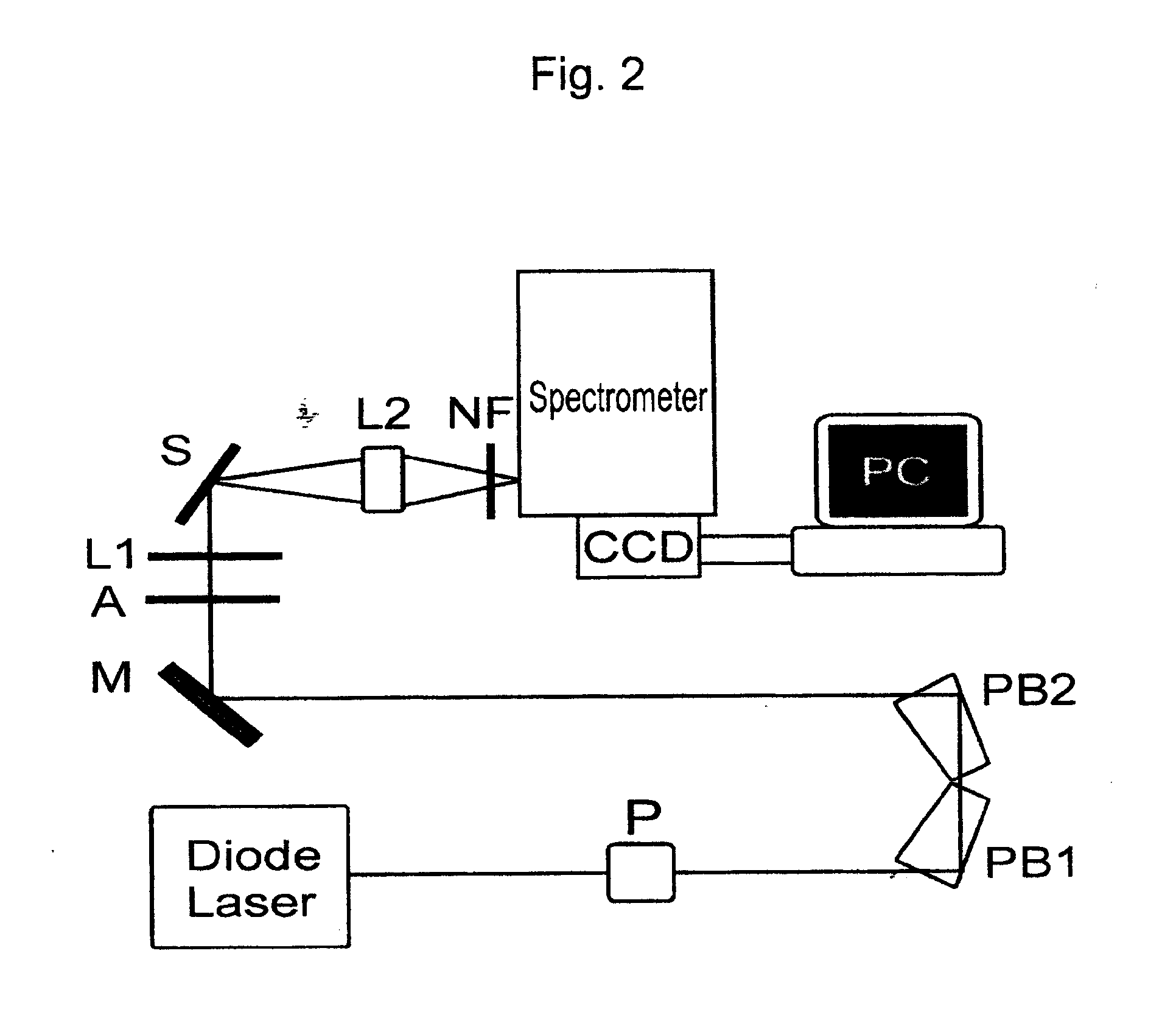
Image
Examples
example 1
Synthesis of Raman-Active Reporter Molecule 4,4′-dithiobis(succinimidylbenzoate)
[0062]The Raman-active reporter molecule 4,4′-dithiobis(succinimidylbenzoate) (DSB) was synthesized following a procedure similar to that used for preparing dithiobis(succinimidylundecanoate) as described in Wagner et al., Biophys. J. 1996, 70, 2052, 15 which is incorporated herein by reference. Briefly, 0.50 g of the reporter molecule dithiobisbenzonic acid (DBA) (1.6 mmol) (Toronto Research Chemicals, Inc), 0.67 g of 1,3dicyclohexylcarbodiimide (DCCD) (3.2 mmol) (obtained from Aldrich), 0.37 g of Nhydroxysuccinimide (NHS) (3.2 mmol) (obtained from Aldrich), and 60 mL of tetrahydrofuran were added to a 100 mL round-bottom flask equipped with a magnetic stir bar and drying tube. The reaction mixture was stirred at room temperature for three days. The solution was then filtered and the solvent was removed under reduced pressure to give an orange residue. The crude product was dissolved in hot acetone and ...
example 2
“Preparation of Raman-Active Reagents Using Co-Immobilization
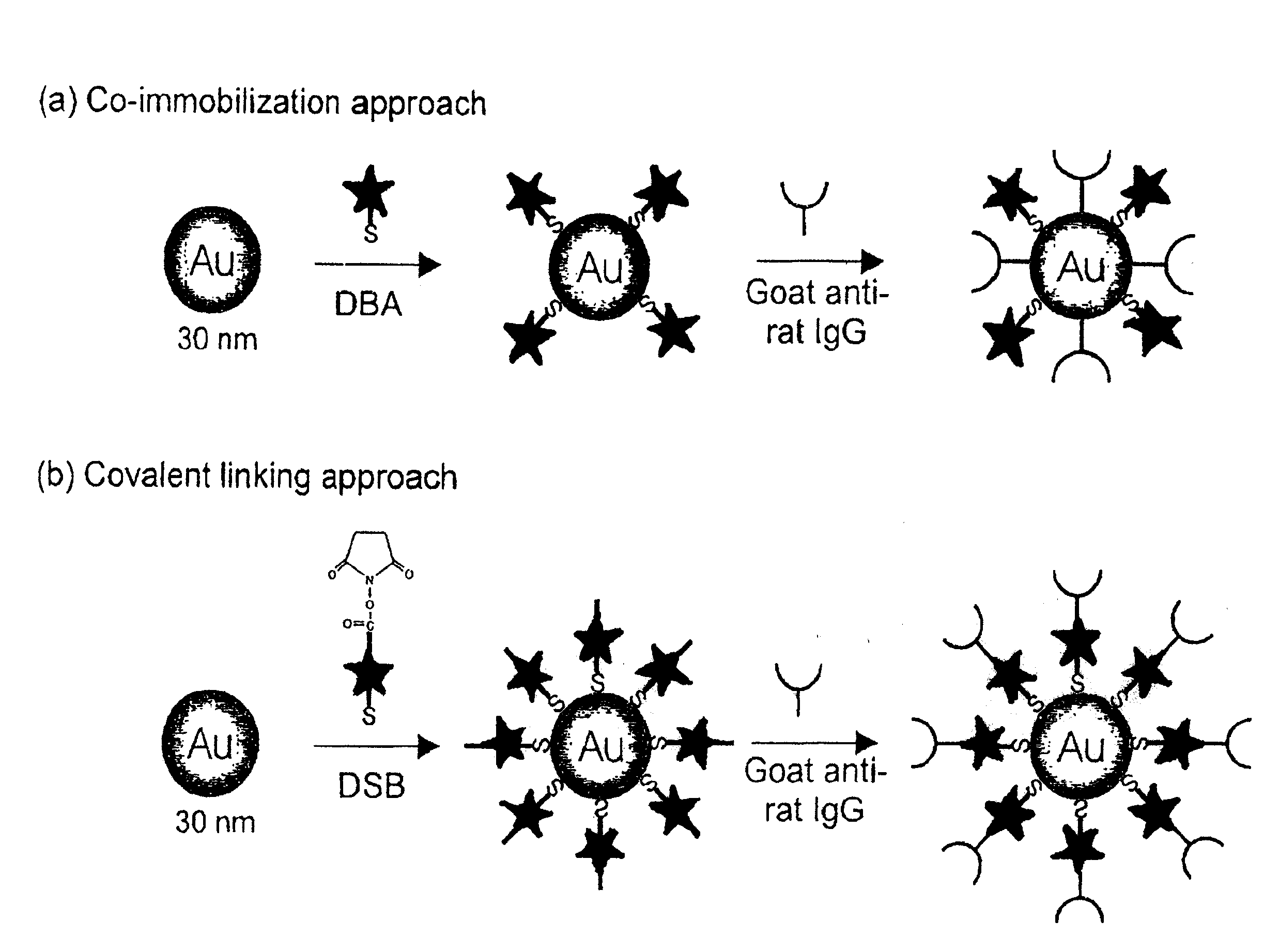
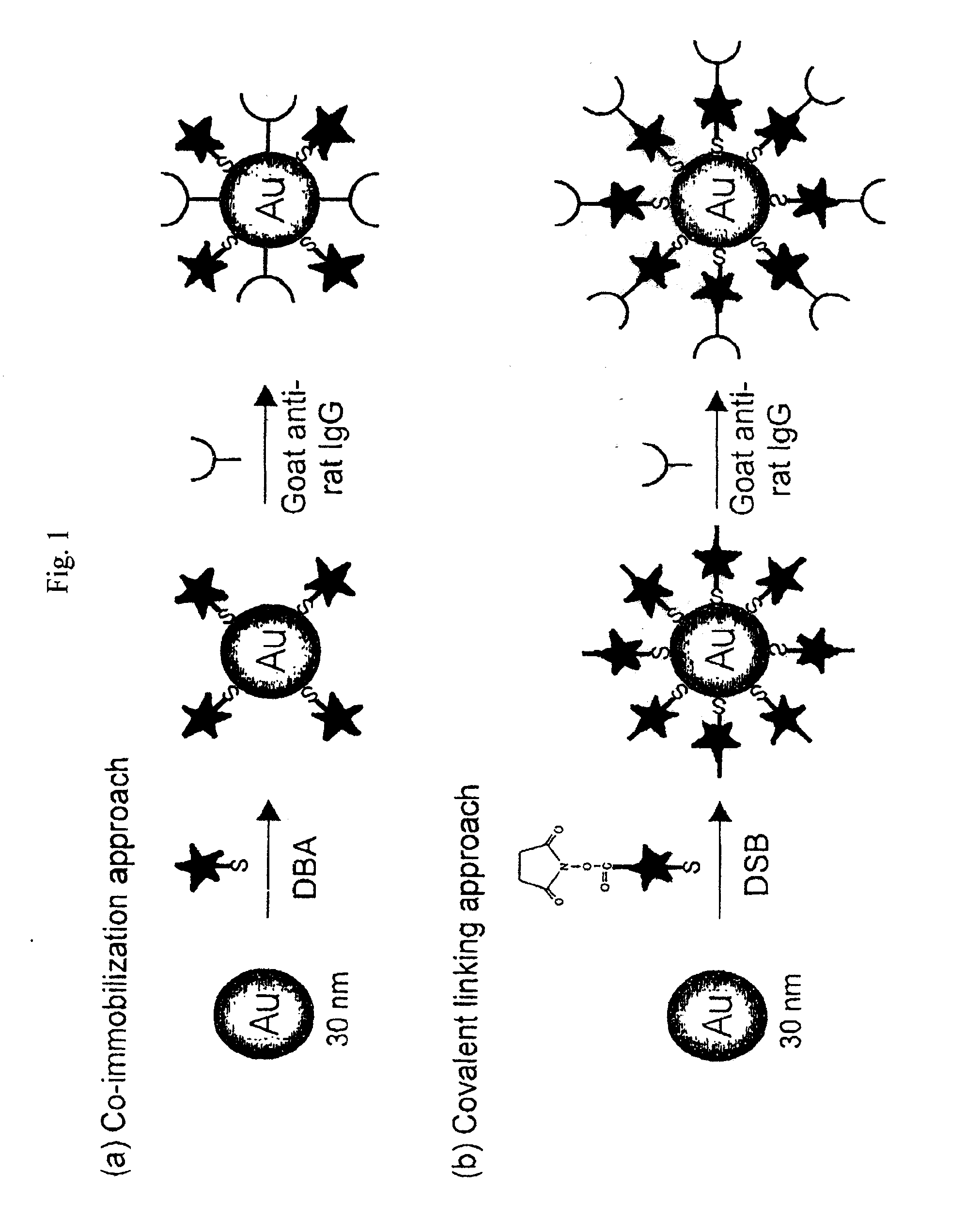
[0063]Raman-active immunogold colloidal reagents were prepared using the co-immobilization approach depicted in FIG. 1(a). First, 25 μL of ethanolic Raman reporter solution (0.5 mM DBA) was added to 10 mL of a suspension of uncoated gold colloids (˜30 nm diameter, 2×1011 particles / mL) (Ted Pella, Inc.). The mixture was allowed to react for 5 hours at room temperature. During this step, the reporter molecules bound via self-assembly onto the colloid surface through the formation of sulfur-gold linkages. We note that this amount of reporter, based on an estimation of the colloidal surface area, will only partially cover the colloid, leaving portions of the uncoated colloidal surface available for protein immobilization. After separating the reporter-labeled colloids from solution by centrifugation at 14,000 g for 4 minutes, the loosely packed, red-colored sediment was resuspended in 10 mL of borate buffer (2 mM, pH 9).
[0064]...
example 3
Preparation of Raman-Active Reagents Using a Covalent Linking
[0065]Raman-active immunogold colloidal reagents were prepared using the covalent linking approach depicted in FIG. 1(b). The DSB molecules were used as both Raman reporters and antibody linkers. The succinimide ester group of the DSB molecule can readily react with the primary amine group of an amino acid, such as the lysine, present in antibodies such as IgG to form a covalent bond. As shown in Scheme 2, the preparation of the covalently-linked colloidal reagent follows a process very similar to that used for the co-immobilized reagents. However, with the covalent linking approach, the antibodies indirectly attached to the colloid through the reporter molecules rather than directly adsorbed onto the colloidal surface. Briefly, 25 μL of a reporter-linker solution (5 mM DSB in CHCl3) was added to 10 mL of bare gold suspension (30 nm) under vigorous agitation. The molecules self-assemble onto the colloid surface, with their...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com