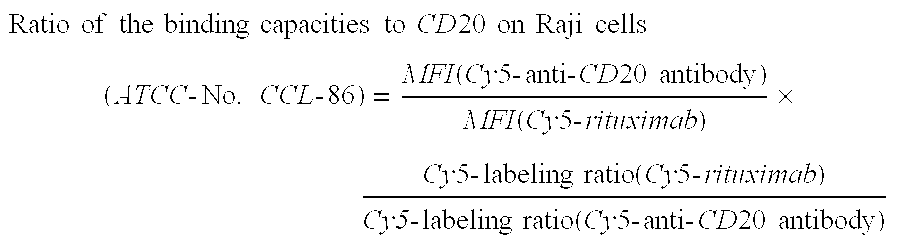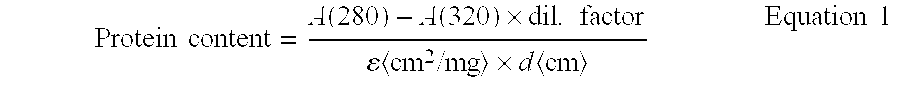Highly Concentrated Pharmaceutical Formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Highly Concentrated Liquid Formulations
[0206]Rituximab is manufactured by techniques generally known from the production of recombinant proteins. A genetically engineered Chinese hamster ovary cell line (CHO) prepared as described in U.S. Pat. No. 7,381,560 is expanded in cell culture from a master cell bank. The Rituximab monoclonal antibody is harvested from the cell culture fluid and purified using immobilized Protein A affinity chromatography, cation exchange chromatography, a filtration step to remove viral contaminations, followed by anion exchange chromatography and an ultrafiltration / diafiltration step.
[0207]rHuPH20 is manufactured by techniques generally known from the production of recombinant proteins. The process begins with thawing of cells from the working cell bank (WCB) or master cell bank (MCB) and expansion through cell culture in a series of spinner flasks. The cell culture up to 6 liters is used to provide a continuous source of cells maintained un...
example 2
Preparation of Humanized 2H7 Anti-CD20 Liquid Formulations
[0226]For the preparation of the liquid formulations, recombinant humanized 2H7 anti-CD20 antibody (2H7.v16 as disclosed in WO 2006 / 084264) was buffer exchanged against a diafiltration buffer containing the anticipated buffer composition and where required, concentrated to an antibody concentration of approx. 60 and 120 mg / ml. After achieving the target concentration, the excipients (e.g. trehalose, rHuPH20, polysorbate 20) were then added as stock solutions to the antibody solution. Finally the protein concentration was adjusted with the final formulation buffer to a humanized 2H7 concentration of approx. 30, 50, and 100 mg / ml.
[0227]All formulations were sterile-filtered through 0.22 μm low protein binding filters and aseptically filled into sterile 3 ml glass vials stoppered with fluoro-resin laminated butyl rubber stoppers and capped with aluminum / plastic flip-off seals. The fill volume was approx. 1.2 ml. These formulatio...
example 3
Treatment of Patients with the Formulation
[0239]Rituximab-containing regimens have become the standard of care for patients suffering from various CD20-positive B-cell malignancies. Currently, rituximab is administered as an intravenous (IV) infusion over several hours. These long infusion times and the side effects related to the infusion were cited by some patients as uncomfortable consequences of the current therapeutic treatment. Furthermore, the required procedure to establish intravenous access is considered invasive and can be painful, particularly in patients with malignant diseases who are treated repeatedly. Subcutaneous (SC) administration could significantly simplify treatment, shortening administration to less than 10 minutes and improving patient experience. Recombinant human hyaluronidase (rHuPH20) has been developed and approved to improve dispersion and absorption of co-administered drugs. It has been combined with rituximab to allow injection volumes larger than 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com