Enhanced Electrode Composition for Li ion Battery
a technology of li ion batteries and electrode compositions, which is applied in the direction of non-metal conductors, cell components, conductors, etc., can solve the problems of poor adhesion of cathode materials to current collectors, broken coatings, and inability to meet the requirements of conventional cathode compositions, so as to achieve enhanced battery performance and less conductive filler loading , the effect of less binder loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Dispersion of Carbon Nanotubes in n-methyl pyrrolidone
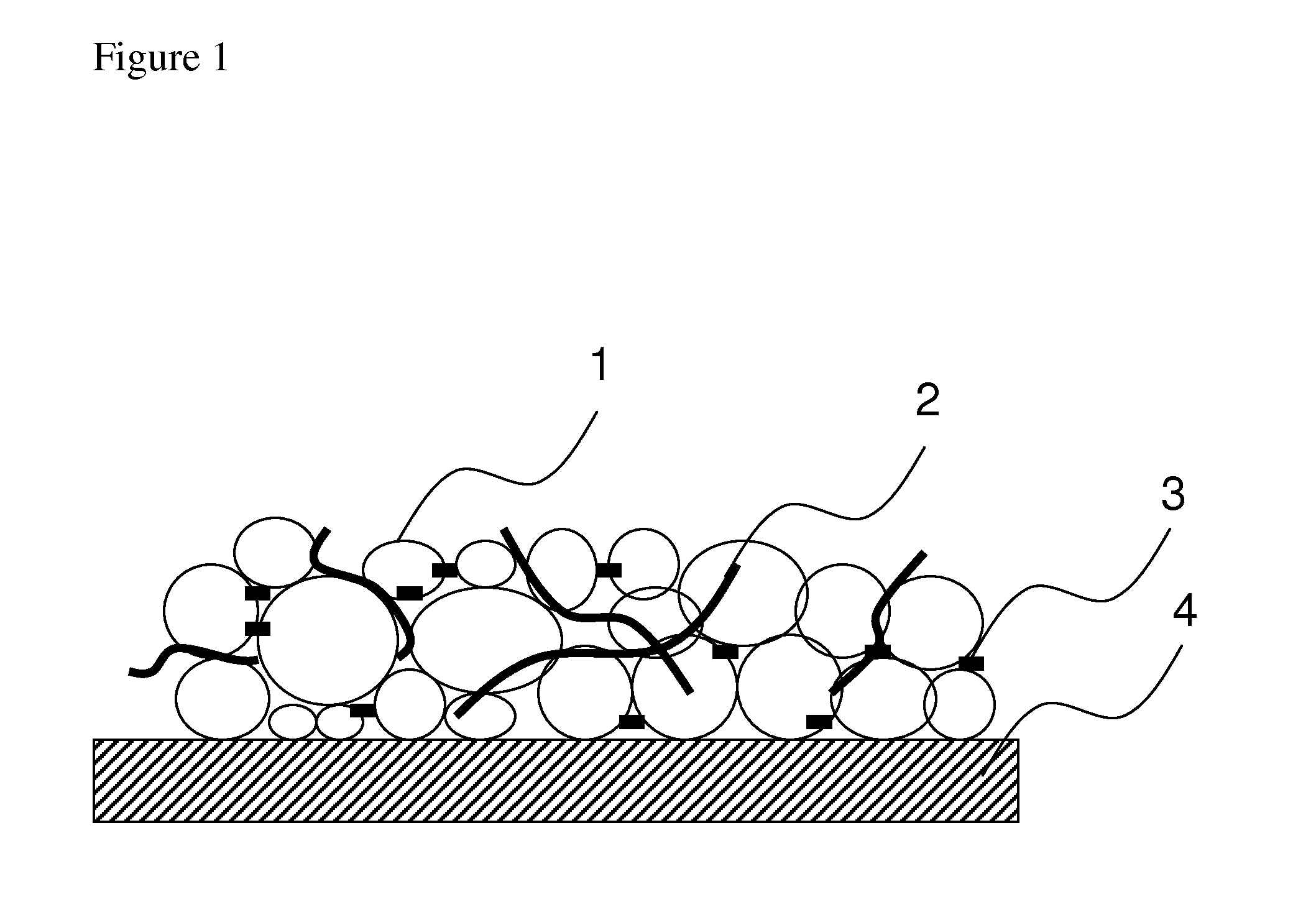
[0047]30 grams of FloTube™ 9000 carbon nanotubes manufactured by CNano Technology Ltd., pulverized by jet-milling, were placed in 2-liter beaker. The tap density of this material is 0.03 g / mL. In another 500 milliliter beaker, 6 grams of PVP k90 (manufactured by BASF) was dissolved in 100 grams of n-methyl pyrrolidone. Then the PVP solution was transferred to the nanotubes together with 864 grams n-methyl pyrrolidone. After being agitated for an hour, the mixture was transferred to a colloid mill and ground at a speed of 3,000 RPM. A test sample was taken out every 30 min. for evaluation. Viscosity was taken at 25° C. using Brookfield viscometer for each sample and recorded; Hegman scale reading was taken simultaneously. Maximum dispersion was observed after milling for 90 minutes. The fineness of this paste reached better than 10 micrometer after 60 minutes of milling. This sample was named as Sample A.
example 2
Electrode Paste Preparation
[0048]A PVDF solution was prepared by placing 10 g of PVDF (HSV900) and 100 g n-methyl pyrrolidone in a 500-mL beaker under constant agitation. After all PVDF was dissolved, designated amount of paste (Sample A) from Example 1 and PVDF solution were mixed under strong agitation of 500-1000 RPM for 30 minutes. The resultant mixture was named Sample B.
[0049]In a separate container, desired weight of active materials such as LiFePO4 or LiCoO3 was weighed under nitrogen blanket. Selected amount of Sample B was also added to the active material and the mixture was stirred under high speed, e.g. 5000-7000 RPM for 5 hours. The resultant viscosity measured by Brookfield Viscometer should be controlled at 3000-8000 cps for LFP, or 7000-15000 cps for LiCoO3. The mixing and stirring was carried out in nitrogen environment and temperature not exceeding 40° C. The resultant sample was named Sample C.
example 3
Electrode Preparation
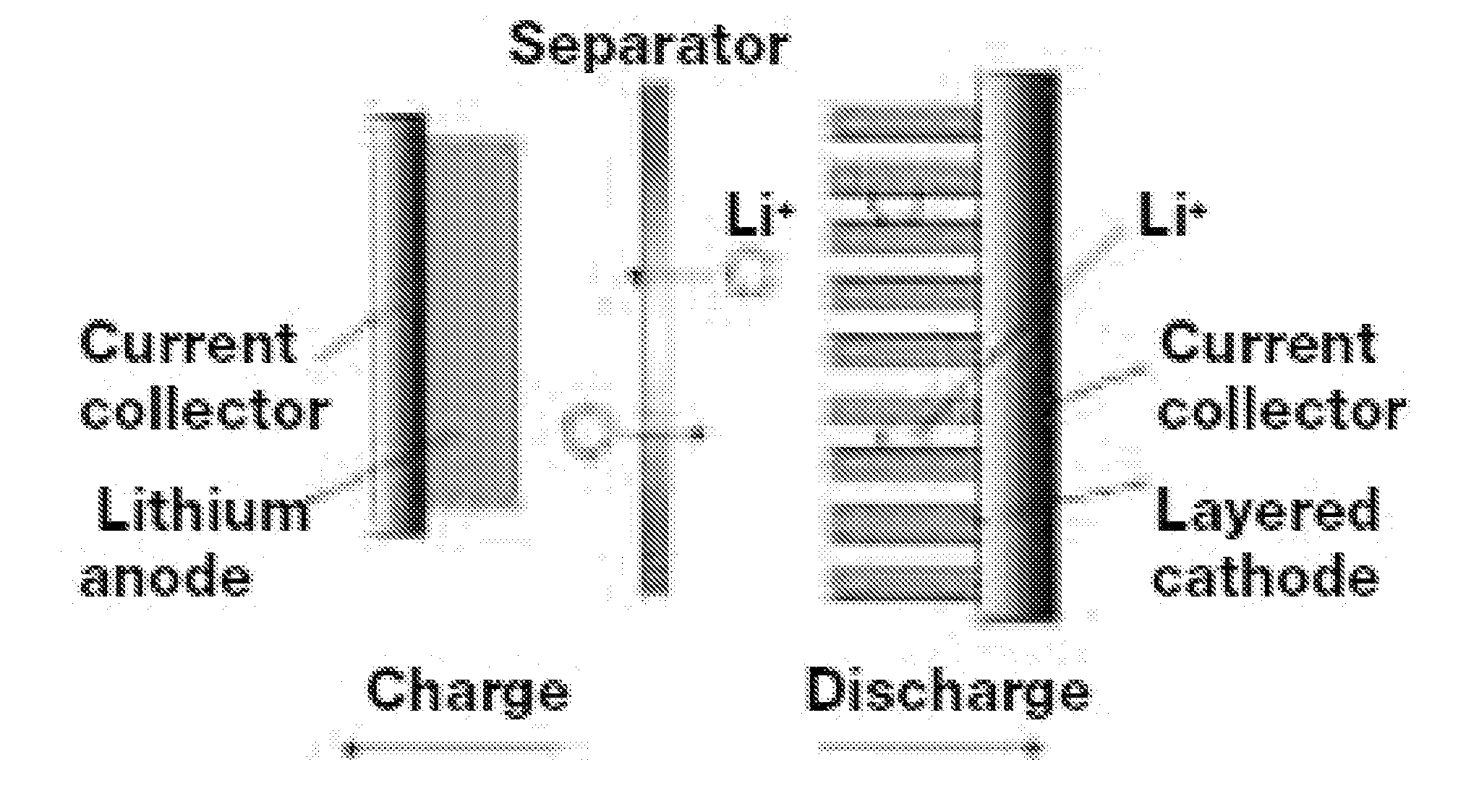
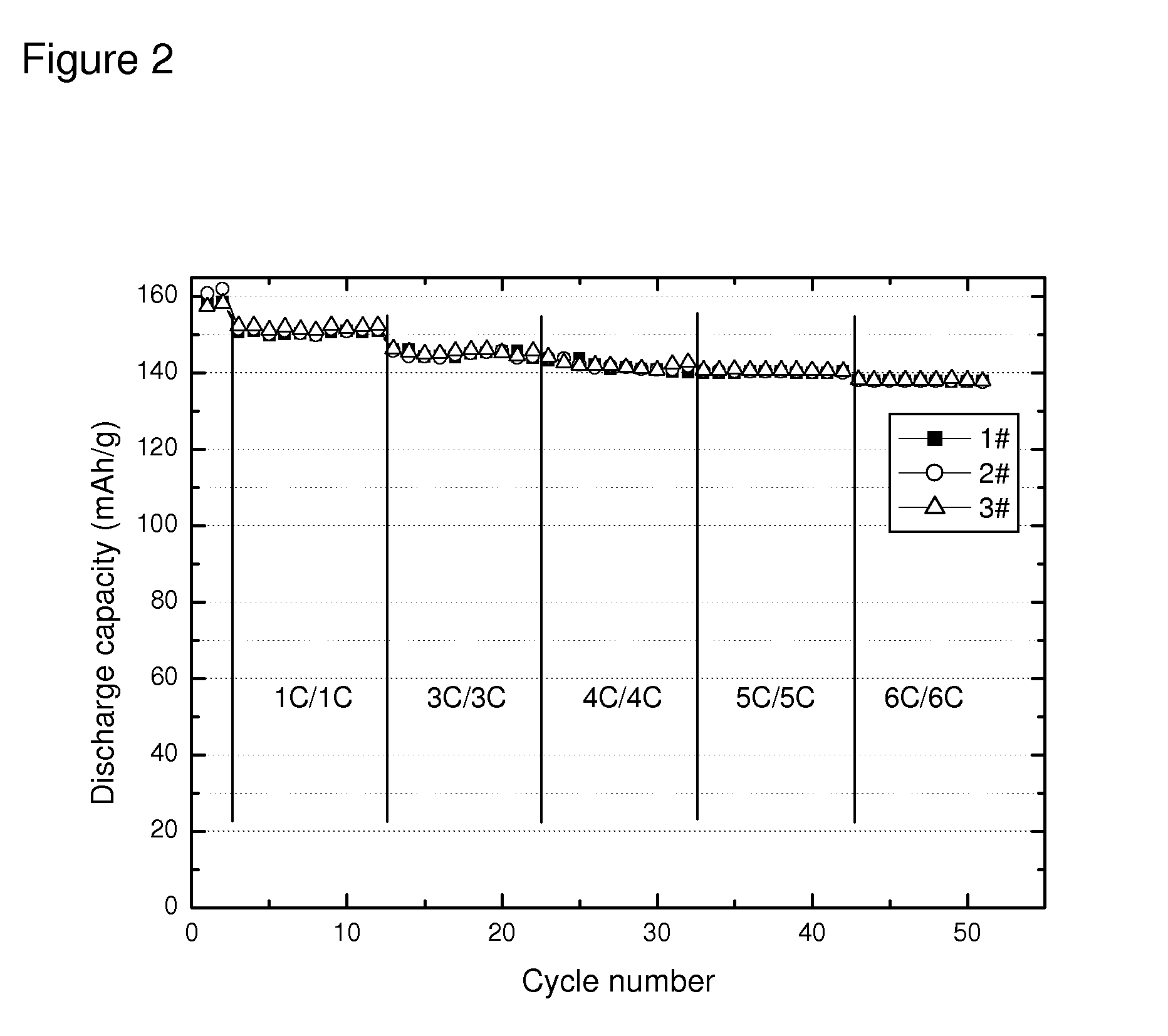
[0050]Clean aluminum foil was chosen as cathode current collector, and placed on a flat plexiglass. A doctor blade was applied to deposit a thin coating of Sample C of thickness of about 40 micrometer on the surface of aluminum foil. The coated foil was then placed in a dry oven at 100° C. for 2 hours. The cathode plate was then roll-pressed to form a sheet. A round disk of coated foil was punched out of the foil and placed in a coin battery cell. Lithium metal was used as anode, and the coin cell was sealed after assemble the cathode / separator / anode and injecting electrolyte. The made battery was then tested for various charging and discharging performance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| tap density | aaaaa | aaaaa |
| interstitial distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com