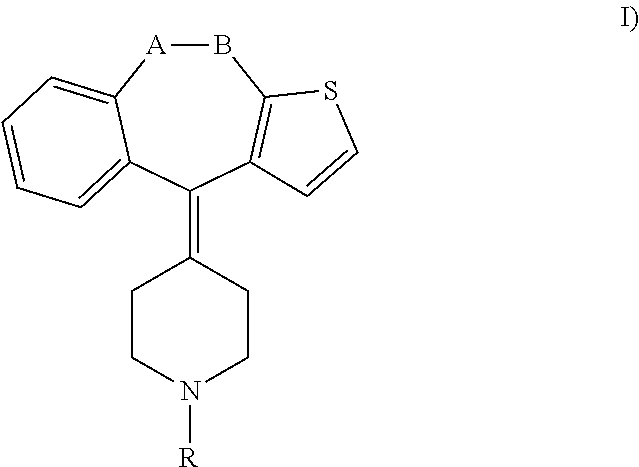
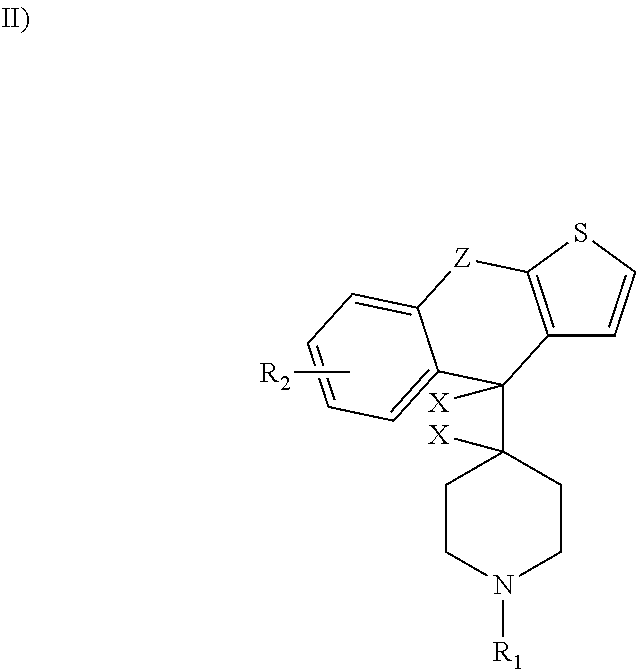
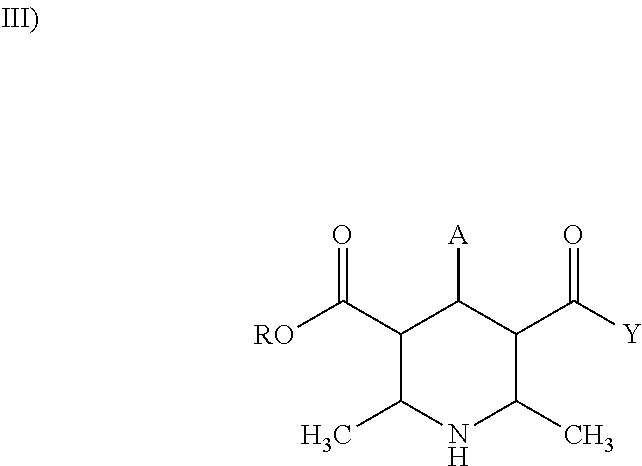
Methods and treatment for allergies and inflammation associated with gastrointestinal diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Use of Ketotifen as a Prophylaxis in Patients at Risk of Sensitization to Hazelnut Allergen
[0783]Twenty-four patients with adverse food reaction are included in this study. As controls, twelve patients matched for age and gender and a history of GERD (Group A) and another twelve patients matched for age and gender and no history of any gastrointestinal disorder (Group B) are included. The history of use of antacids and satisfaction are recorded for both groups.
[0784]To analyze hypersensitivity to hazelnut, a double-blind, randomized, dose-ranging study is carried out with patients with a history of hazelnut allergy manifested by urticaria, angioedema, respiratory tract symptoms or hypotension generally using the methods described in Israel et al., Am. J. Respir. Crit. Care Med., (2000) 164:75-80. Eligible patients include those with serum total IgE level of 30-1000 IU / mL and / or a positive skin-prick test to hazelnut. Patients must have asthma condition under control with a forced ex...
example 2
Use of Ketotifen During Desensitization to Airborne Allergen
[0790]To evaluate the effectiveness of mast cell stabilizer, e.g., ketotifen, in desensitization or immunotherapy, particularly RUSH immunotherapy, a randomized, double-blind, placebo-controlled study is performed. Patients are randomly assigned to 4 treatment groups (1:1:1:1). Pretreatment with either ketotifen, 1 mg, b.i.d., or placebo, is carried out for nine weeks to protect, prevent, inhibit or ameliorate allergic reaction, particularly to prevent, normalize or ameliorate gastrointestinal symptoms. One-day rush immunotherapy (week 0) is completed at least 3 weeks before the start of the ragweed season. On the day of rush immunotherapy, the patients receive 6 injections of either placebo or aqueous short ragweed extract. Ragweed dosing starts with a diluted extract containing 0.012 μg of Amb a 1 (a major allergen in ragweed) over a 3-hour period, reaching a maximum of 1.2 μg of Amb a 1. Some subjects also receive 2 addi...
example 3
Use of Ketotifen During Desensitization to Oral Allergen
[0793]To evaluate the efficacy of ketotifen in desensitization process against oral allergen such as hazelnut, a similar study as described in Example 3 may be carried out except with the use of hazelnut extract rather than ragweed extract. The studies will show that patients who receive ketotifen plus immunotherapy have fewer adverse events of gastrointestinal disorders and significantly improved allergy severity scores compared to those receiving immunotherapy alone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com