Bismuth metal oxide pyrochlores as electrode materials for electrolytic ozone and perchlorate generation
a technology of ozone and perchlorate, which is applied in the direction of electrolysis components, instruments, optics, etc., can solve the problems of low process efficiency, low concentration of ozone in water, and increase power consumption, so as to achieve safe and effective replacement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
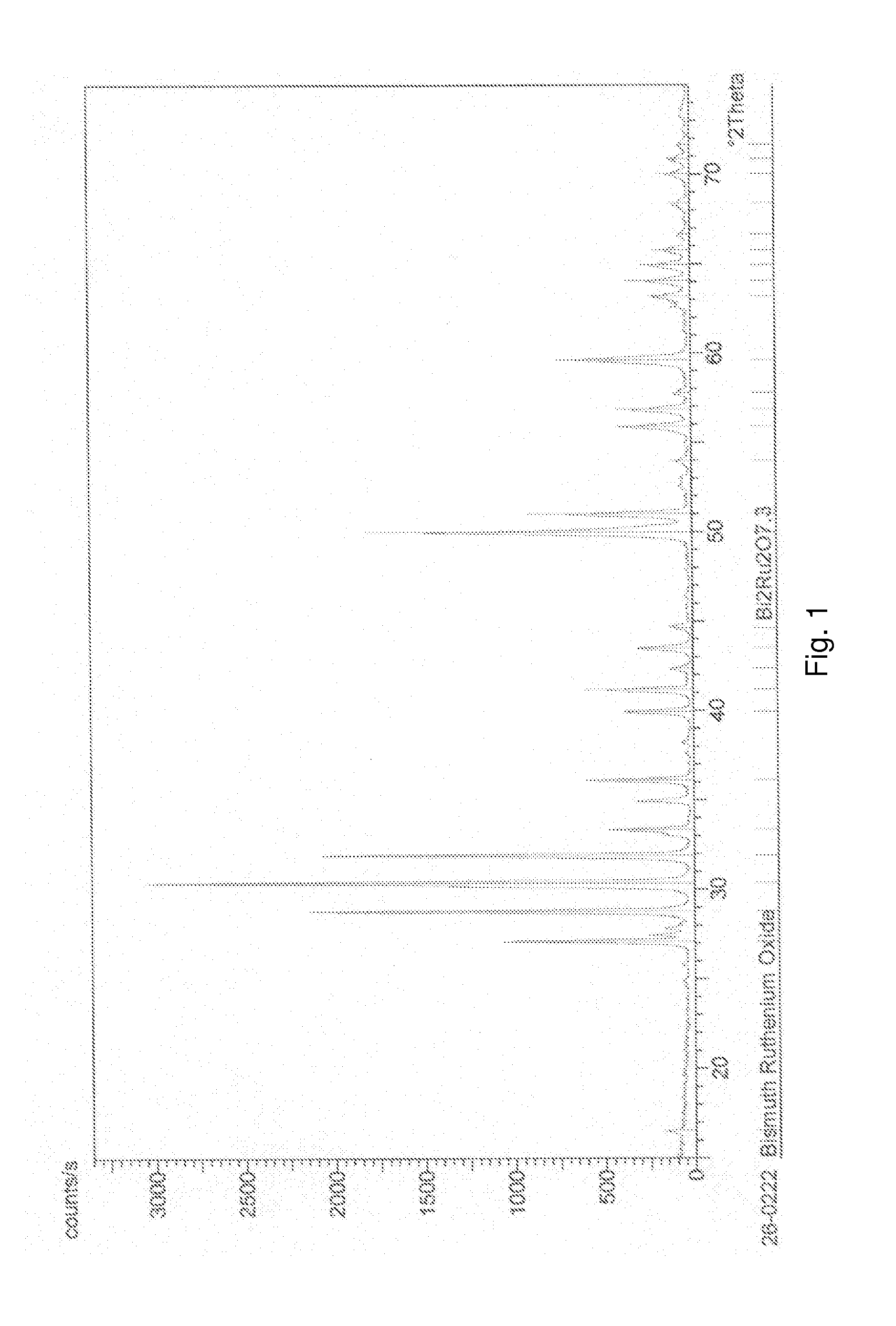
[0052]Synthesis of Bi2Ru2O7. Stochiometric amounts of Bi2O3 and RuO2.xH2O (the concentration of RuO2 and H2O were determined by thermal gravimetric analysis (TGA) to be 76% and 24% respectively) were made into slurry by adding water and mixing with mortar and pestle. After mixing for 15 minutes, a consistent slurry was formed. The slurry was then dried in a drying oven and again mixed with pestle before heating at 650° C. for 24 hours followed by 750° C. for 24 hours. X-ray diffraction (XRD) was performed at each stage to determine the phase purity of the product. The XRD results are shown in FIG. 1. XRD data after the 750° C. step matched well with the Joint Committee on Powder Diffraction Standards (JCPDS) reference pattern with Bi2Ru2O7.3 except for 2 peaks. These peaks could not be attributed to either of the starting materials Bi2O3 and RuO2. Additional heating at 750° C. for another 24 hours did not change the intensity of the two peaks. Hence it was concluded that these peaks...
example 2
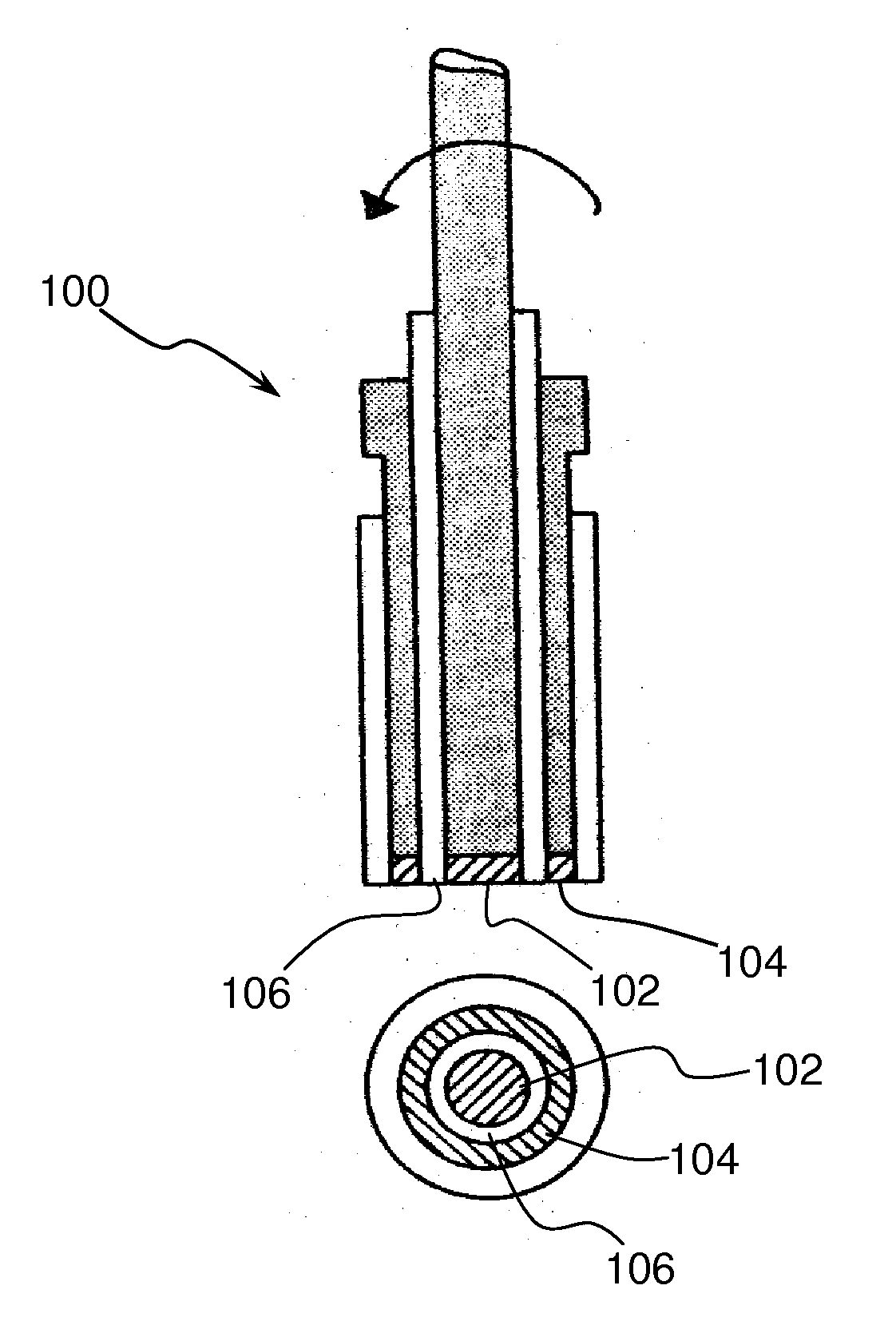
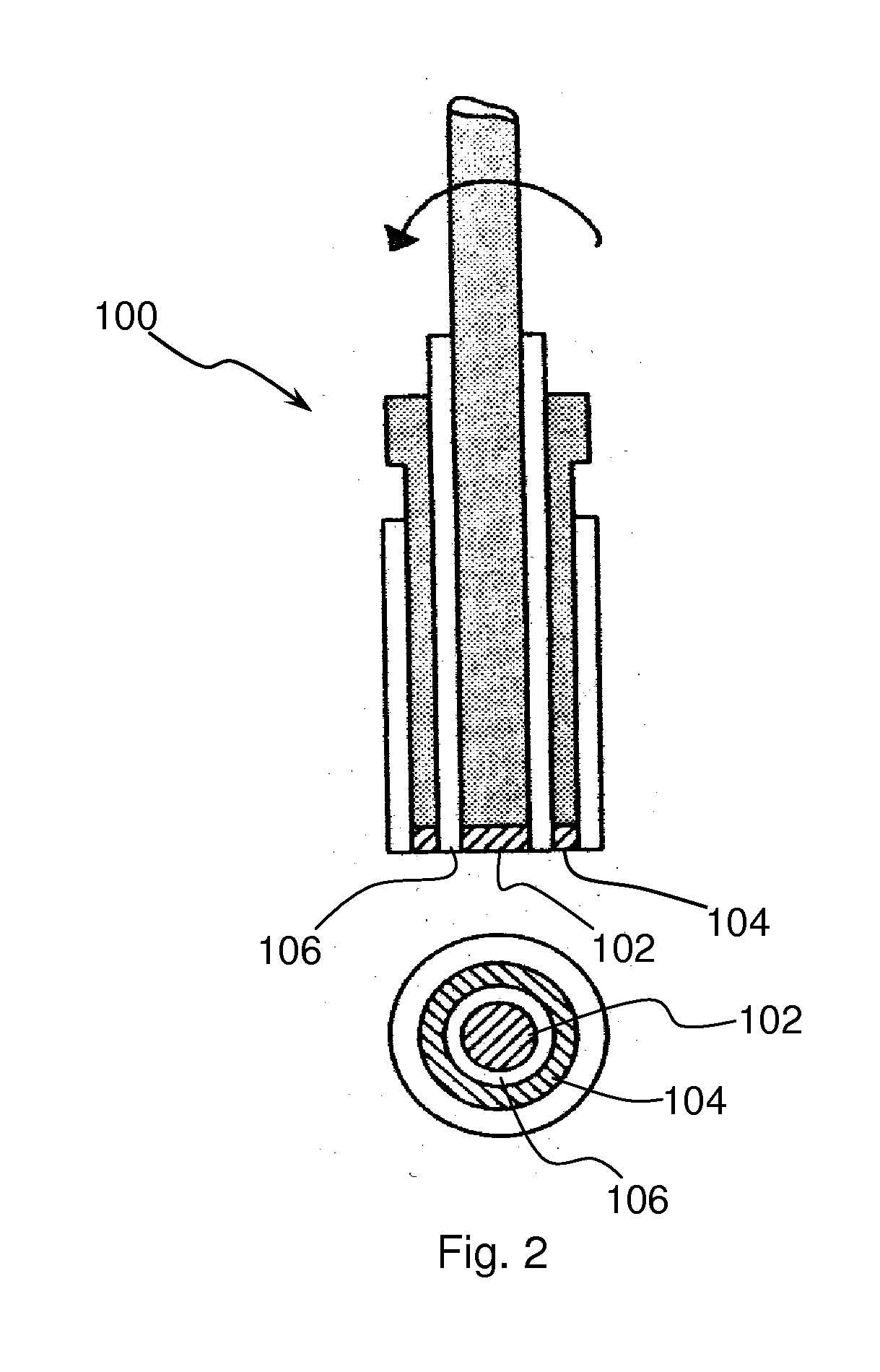
[0053]Rotating Ring Disk Electrode for Ozone analysis. A Rotating Ring Disk Electrode (RRDE) method was used to demonstrate that bismuth ruthenate can indeed function as an anode in electrolytic ozonator. A cross-sectional side view and bottom view of a typical RRDE device is shown in FIG. 2. The theory behind the application of RRDE for in-situ generation / detection of ozone is briefly as follows. The RRDE 100 consists of a central disk electrode 102 surrounded by a concentric ring electrode 104 with a thin Teflon U-cup insulator 106 separating them. The potential or the current at each electrode can be controlled independently using a bipotentiostat (not-shown). A bipotentiostat controls the voltage and measures the current at two working electrodes immersed in an electrolyte, using only one reference electrode and one counter electrode. The RRDE shown in FIG. 2 can be used to detect and measure O3 that is generated at the disk of RRDE. When the central disk electrode 102 is anodic...
example 3
[0057]Preparation of the Bi2Ru2O7 RRDE disk. The synthesized Bi2Ru2O7 product from Example 1 was mixed with aqueous suspension of polyvinyl alcohol binder to coat the particles with the binder. The Bi2Ru2O7 material and binder were pressed into pellets and fired at 1100° C. in air for 24 hours. The XRD pattern for the sintered material is shown in FIG. 3. The XRD pattern indicates that the crystal structure of the sintered material is quite different from the starting material. The identity of this material phase is not presently known. The final diameter of the pellets was ˜0.6 mm. The pellets were further ground to the size and shape of the RRDE disk for use in the RRDE setup. The surface of the disk was also polished to further smooth the surface. Conductive silver paint was applied on the back side of the disk and cured at 700° C. This was done to ensure good electrical contact between the metal spring of the RRDE and the mixed metal oxide disk. Scanning electron micrograph (SEM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com