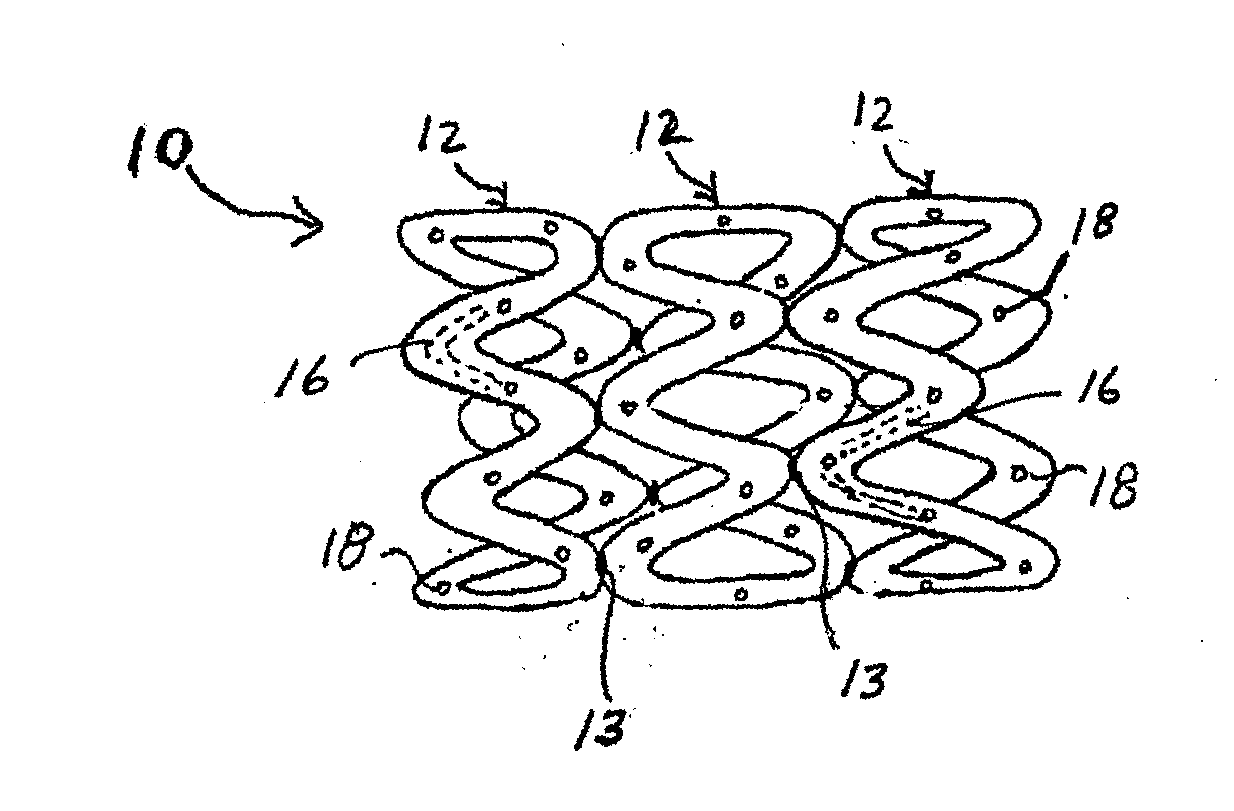
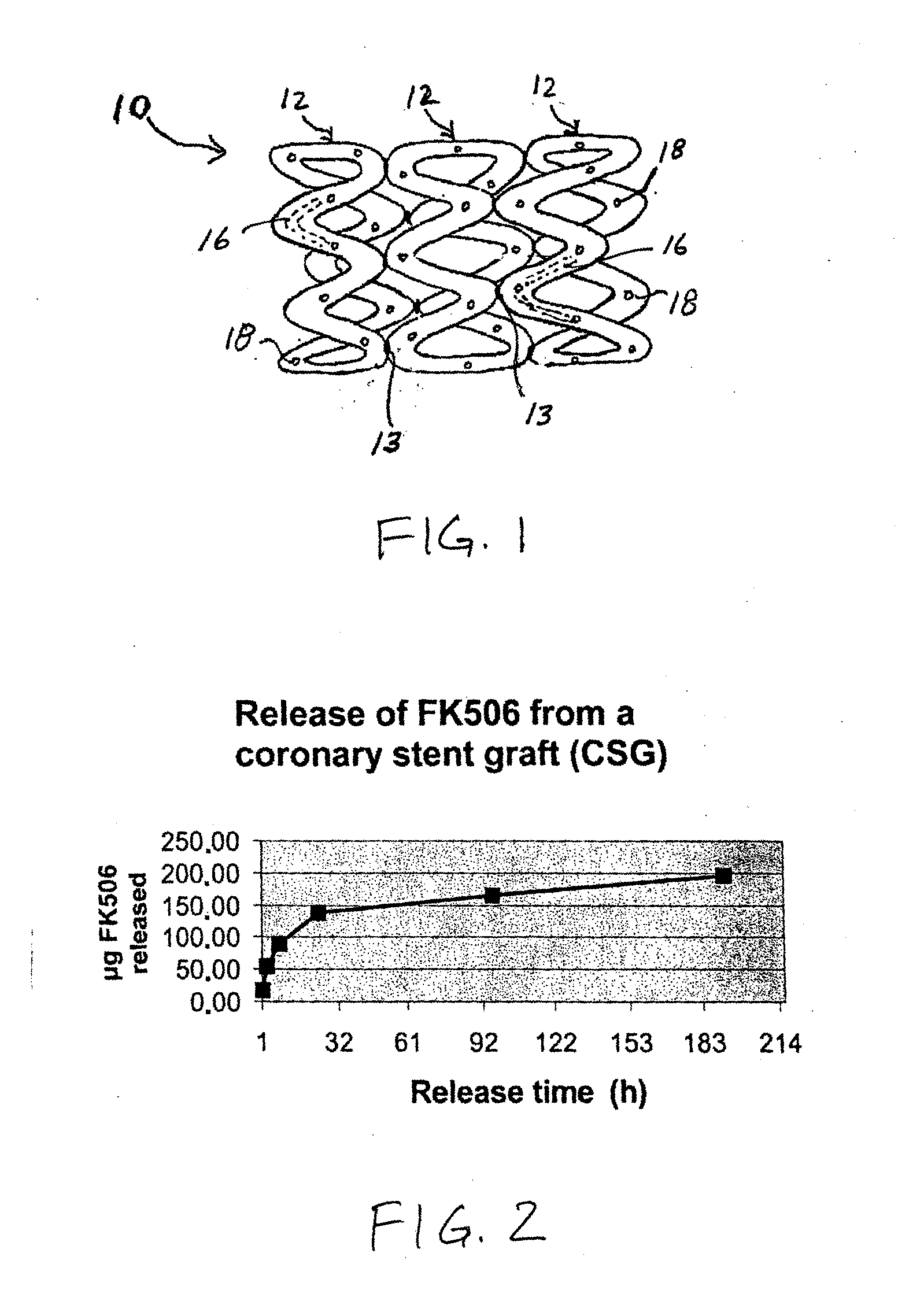
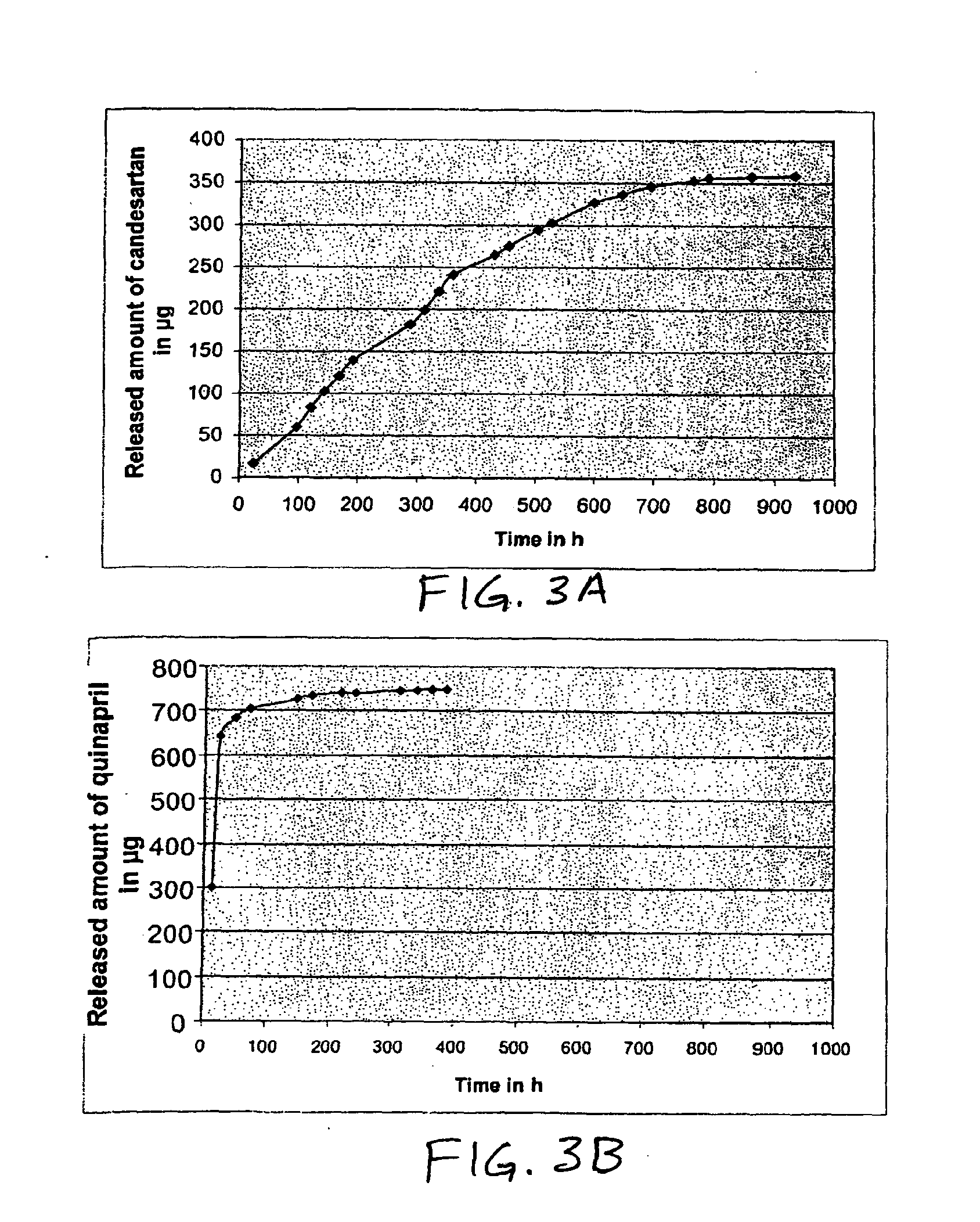
Medical implants containing fk506 (tacrolimus) methods of making and methods of use thereof
a technology of tacrolimus and implants, which is applied in the field of implants, can solve the problems of affecting affecting the quality of the implant, and affecting the quality of the implant, and none of the active agents have been able to reduce the restnosis significantly, and agents delay the healing of the vessel wall injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0105]Loading of Selected Active Agents onto Stent Grafts
[0106]All values are stated in μg.
TABLE 1Active agentFK 506Pac-type of(tacro-Vin-litaxelCis-Mitoxan-stentlimus)blastine(Taxol)platintroneA1382412815*116167112515514*142162523814814*113Average155914614414 124B181621Average18C194165Average180*measured by AASA: Experiments with dissolved solids in which stent grafts with a PTFE polymer layer were immersed in the solution.B: Experiments with i.v. solutions in which stent grafts with a PTFE polymer layer were immersed in the solution.C: Experiments with i.v. solutions in which polyurethane-coated stents were immersed in the solution.
example 2
Release Patterns of Various Stent Grafts and Stents Having Polymeric Coating of the Present Invention:
[0107]All values are stated in μg.
[0108]To analyze the active agent release, a stent was incubated at 37° C. in 10 ml of PBS buffer (stabilized with Na azide) at 37° C. After defined periods of time had elapsed, 2×1 ml of the solution were removed and analyzed. These 2 ml were replaced by fresh PBS buffer (stabilized with Na azide).
[0109]The tables reflect the total released content of active agent in the solution., ie., the amounts of active agent in the buffer volume removed for the analysis are accumulated with amounts detected in subsequent samples.
TABLE 2ActiveVinblastineagent / after 96 hrTypeafter 1 hrafter 3 hrafter 8 hrafter 24 hrafter 72 hrA73 and108 and121 and106 and132 and75114126120140Average:Average:Average:Average:(96 hr)74109124113Average:13637 and48 and47 and57 and56 and4151586257Average:Average:Average:Average:(72 hr)39504260Average:5780 and99 and108 and117 and113 an...
example 3
Production Process (1) for FK506-Coated Stents:
[0110]10 mg of FK506 are dissolved in 3 ml of ethanol.[0111]Uncoated stainless steel stents are immersed in the solution at room temperature under vacuum overnight.[0112]Wash three times with saline for 1 minute.[0113]Dry overnight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com