Composition for amelioration of hypoalbuminemia
a technology of hypoalbuminemia and composition, which is applied in the field of composition for enhancing hypoalbuminemia, can solve the problems of reducing compliance, nausea, diarrhea, and reducing the effect of albumin production, and achieving the effects of reducing side effects, reducing side effects, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0045]Now, the present invention will be more specifically described based on Examples; however, the present invention is not restrictively interpreted based on the description of these Examples.
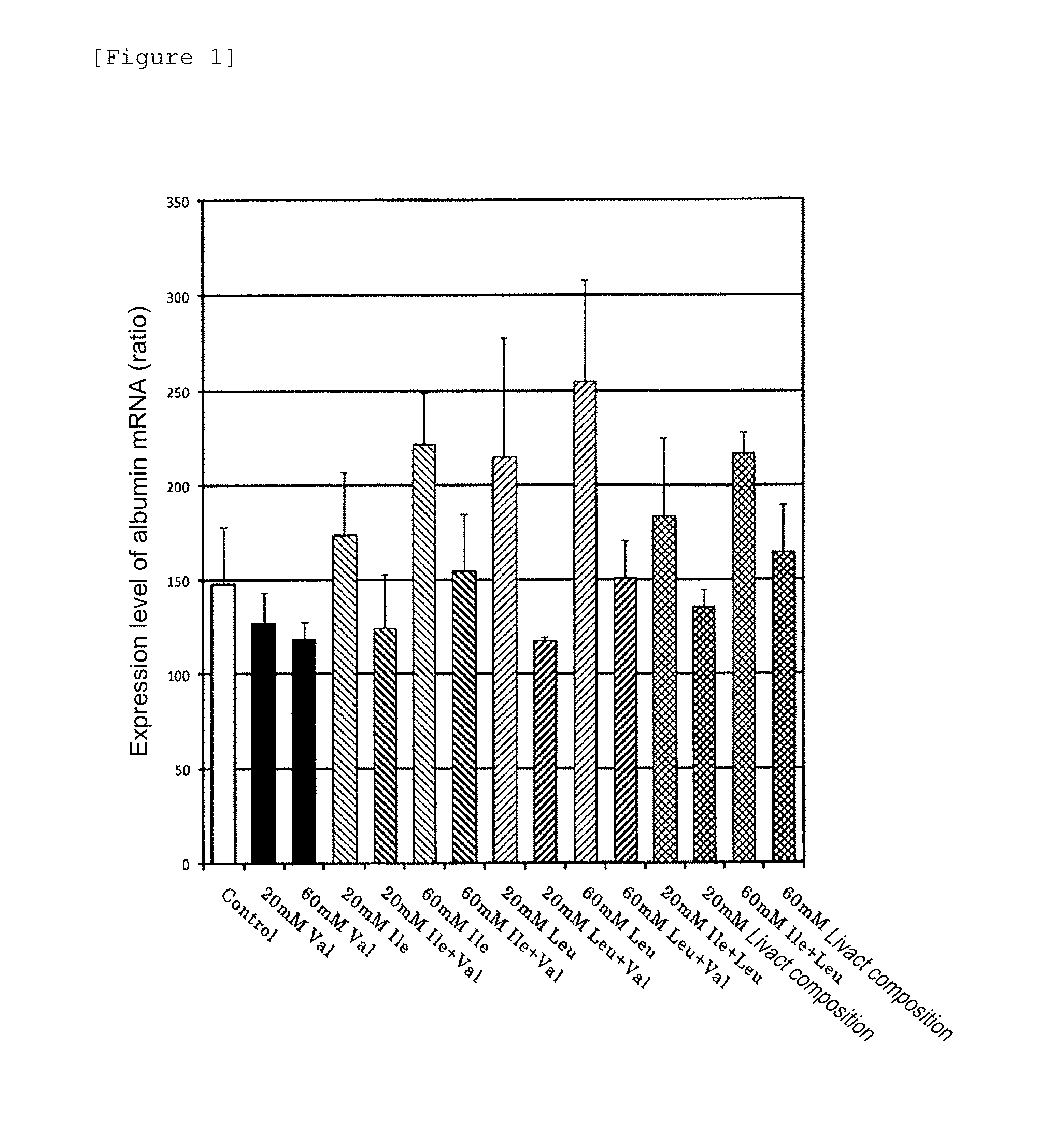
[0046]In this test, human primary cultured hepatocytes were cultured by each test solution to check a change of albumin mRNA expressional potency and a change of secretion level of albumin. The test was performed as follows.
[0047](1) Albumin mRNA and Hypoxanthine Phosphoribosyltransferase 1 (Hereinafter Referred to Simply as “HPRT1”) mRNA
[0048]Albumin mRNA and HPRT1 mRNA were measured. The nucleotide sequences of albumin and HPRT1 are registered in the GenBank as follows and each nucleotide sequence follows the sequence registered.
[0049]Albumin; GenBank accession number XM 031322
[0050]HPRT1; GenBank accession number NM 000194
[0051]Note that, HPRT1, which is a house keeping gene serving as a control, was measured in the same test. The sequences of primers and probes used in measurement of alb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass ratio | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com