Nano-Hybrid Delivery System for Sequential Utilization of Passive and Active Targeting
a delivery system and hybrid technology, applied in the direction of nanocapsules, pharmaceutical delivery mechanisms, capsule delivery, etc., can solve the problems of preventing the clinical translation of polycations in drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0054]Materials.
[0055]Branched PEI (Mn 10,000), PLGA (50:50, Mw 40,000-75,000), poly(vinyl alcohol) (PVA, 87-89% hydrolyzed, Mw 13,000-23,000), rhodamine B isothiocyanate (RITC, mixed isomers), dichloromethane (DCM), pyridine, p-nitrophenyl chloroformate (p-NPC), triethyleamine (TEA), diethyl ether, and cholesterol were all obtained from Sigma-Aldrich (St. Louis, Mo.). Amine-terminated methoxy PEG (mPEG-NH2) (Mw 5,000) was obtained from Nektar (Huntsville, Ala.). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-mPEG-2000 (DSPE-PEG 2000), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), and 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) sodium salt (DOPG) were purchased from Avanti Polar Lipids Inc. (Alabaster, Ala.). All other chemicals used in this study were purchased from Sigma-Aldrich unless specified otherwise.
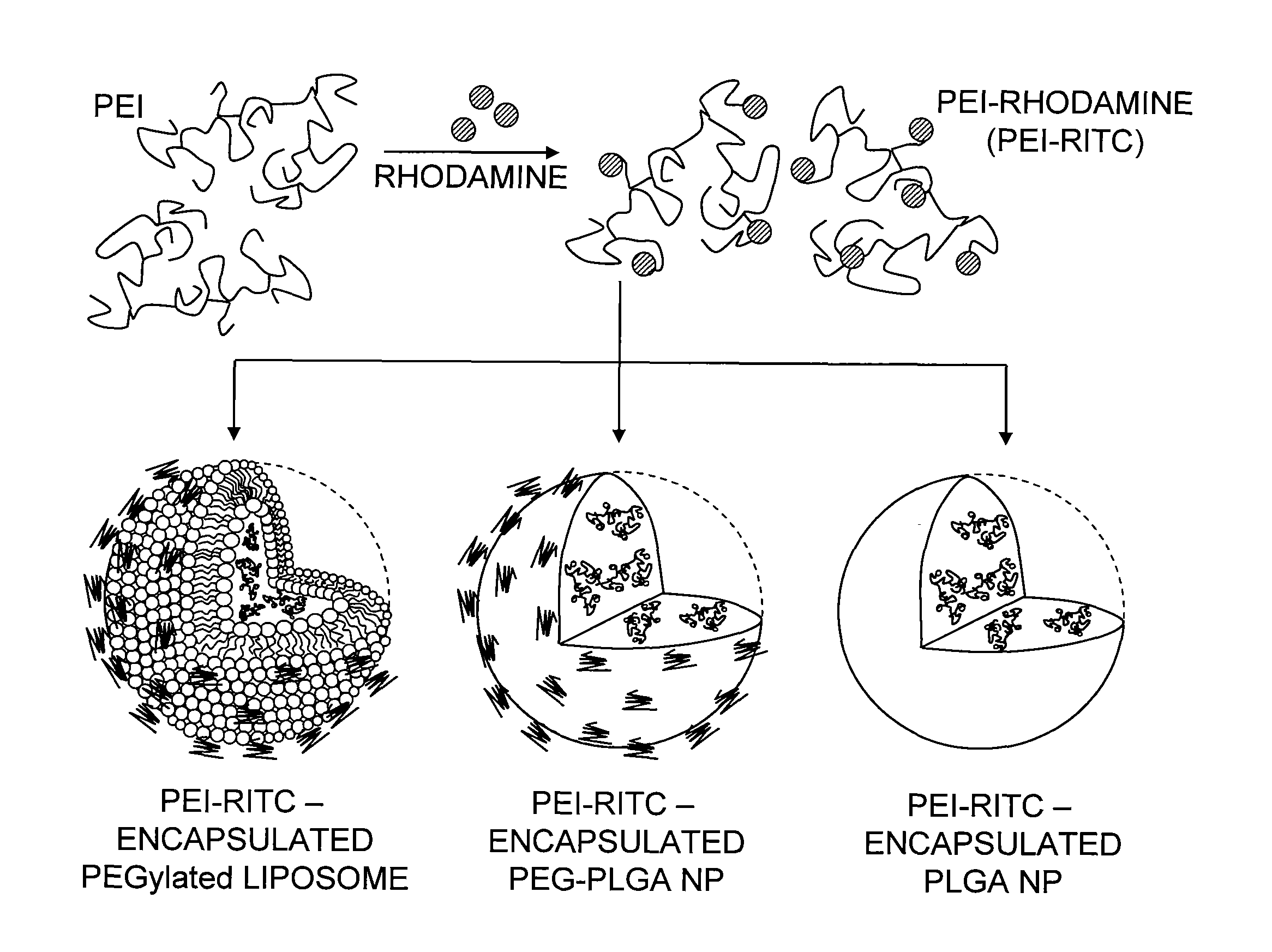
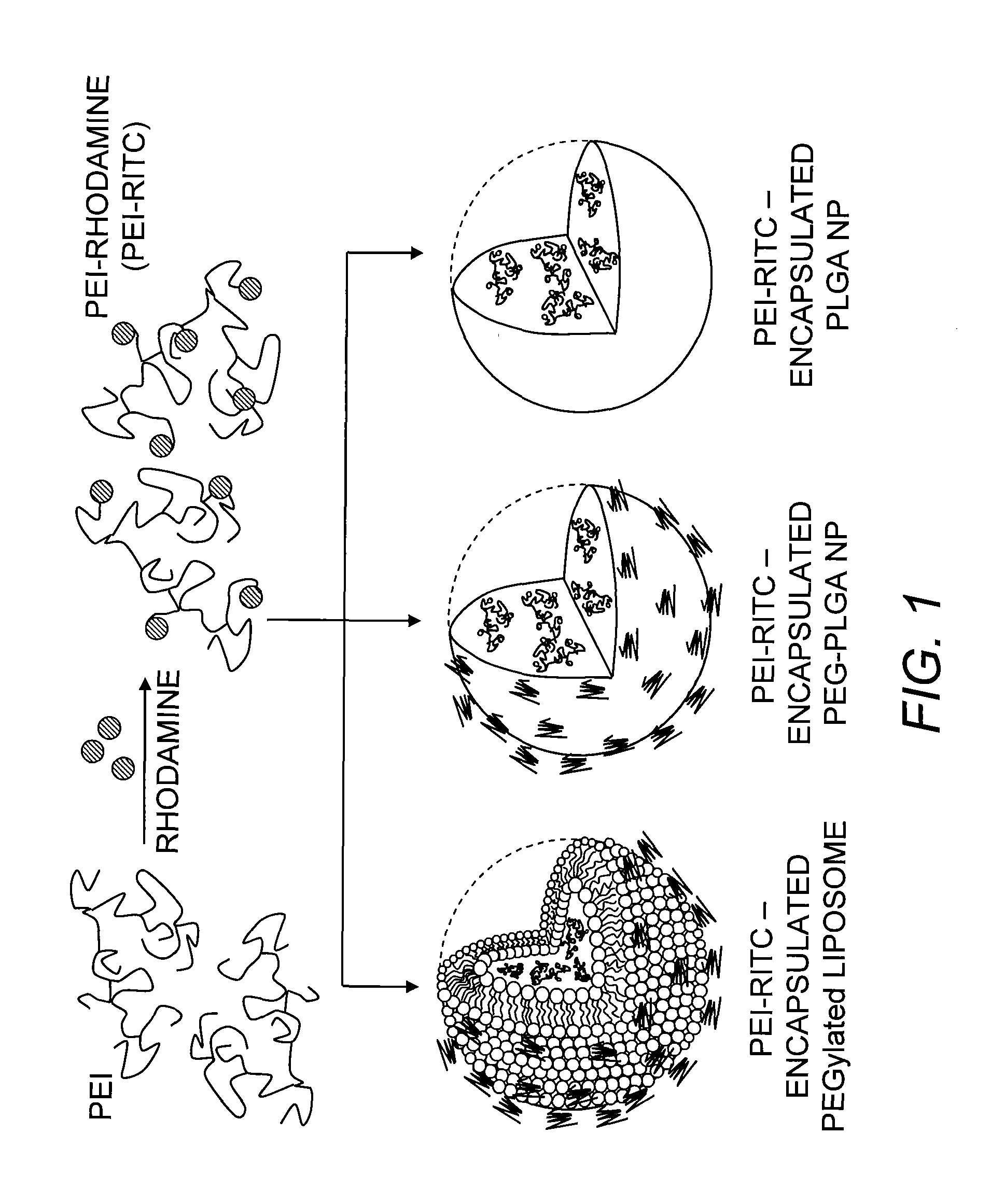
[0056]Preparation and Characterization of PEI-RITC Conjugates.
[0057]PEI was fluorescently labeled by conjugation with RITC using a similar me...
example 2
Preparation and Characterization of PEI-RITC Conjugates and PEG-PLGA Copolymer
[0082]The UV / Vis measurements revealed the number of RITC molecules attached to a PEI chain. By constructing a calibration curve of UV absorbance of RITC against various concentrations at 555 nm (λmax) the RITC concentration in a solution of the PEI-RITC conjugate was calculated from the absorbance at 555 nm. The molar ratio of PEI and RITC was then calculated by converting the concentration values to number of moles. The results indicated the presence of 6.2 RITC molecules per PEI chain. Particle size and zeta potential of the conjugates were measured to be 11.2 nm and 32.1 mV, respectively (Table 1). In addition, the chemical structure of PEG-PLGA was confirmed by 1H NMR. On the basis of the relative integration values of the characteristic peaks of each polymer, the ratio of the PEG block to the PLGA block was measured to be 1.3:1-2.4:1.
TABLE 1LoadingZeta(μg / mgLoadingParticlePotentialpolymerEfficiencyFo...
example 3
Preparation and Characterization of PEI-RITC-Encapsulated Nanohybrids
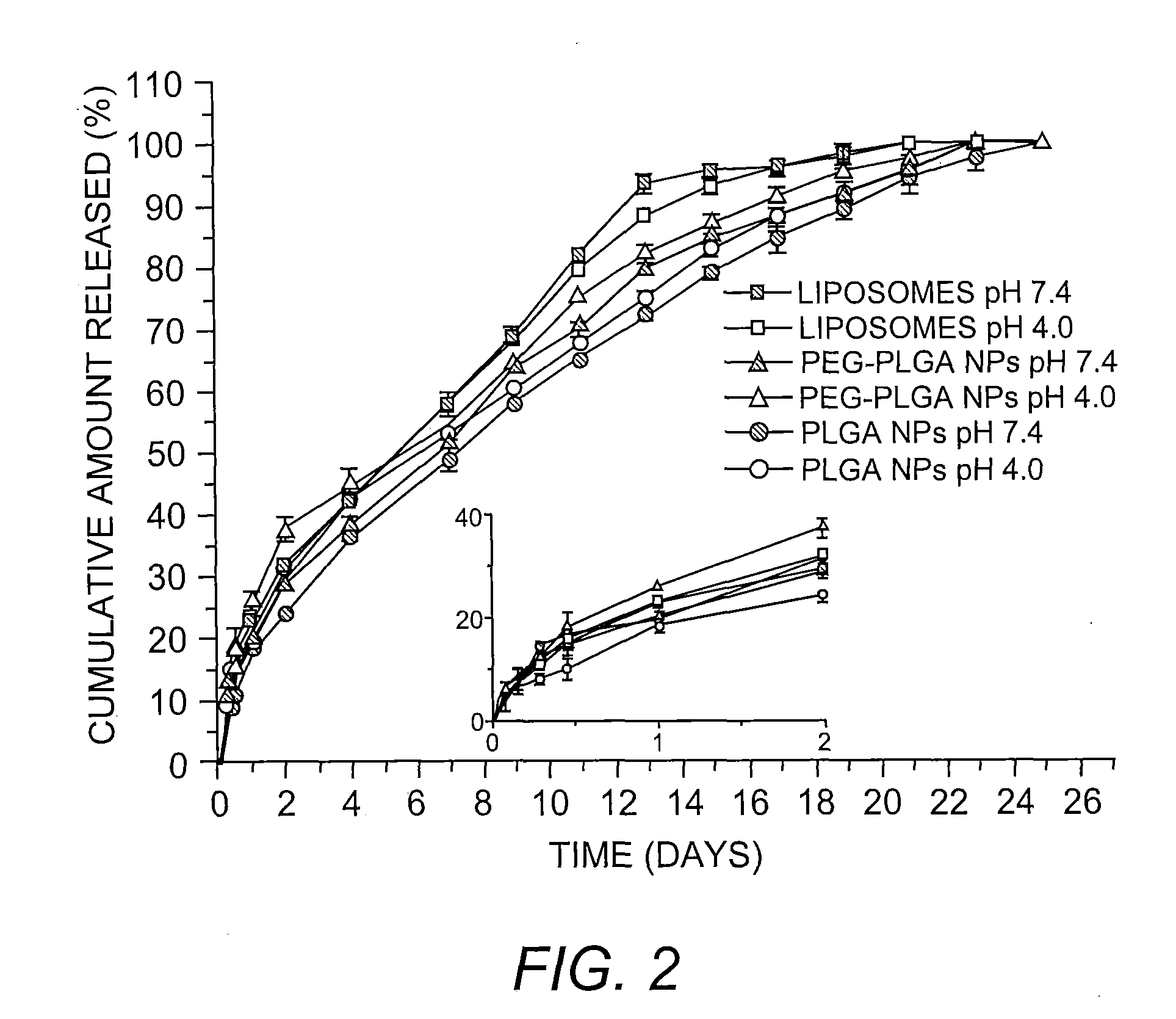
[0083]It is highly desirable for a potential tumor-targeted delivery system to possess a size range of less than 200 nm in order to be able to passively accumulate into the tumor tissue. The size of the liposome-based system was controlled by extrusion using a membrane filter with pore size of 100 nm according to a slightly modified method previously described (Ko, et al. (2009) supra). For encapsulation into the polymeric NPs, the double emulsion method was chosen as it enables encapsulation of hydrophilic materials into a variety of polymers or copolymers with a controlled particle size (Perez, et al. (2001) supra; Vauthier & Bouchemal (2009) Pharm. Res. 26:1025-1058). As shown in Table 1, the encapsulation methods employed herein were successful in controlling the size, as particle sizes for all three nanohybrids were in the range of 100-150 nm. Both methods also showed relatively good loading efficiencies (45-9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
| Solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com