Synthetic luciferase gene and protein
a luciferase gene and synthetic technology, applied in the direction of peptides, drug compositions, immunological disorders, etc., can solve the problems that current luciferases isolated from various organisms, including insects and marine organisms, are not necessarily optimized for expression or production, and achieve the effect of improving thermostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthetic Construction of the COS Luciferase Gene
[0071]The synthetic COS luciferase gene, SEQ ID NO:3, was assembled from synthetic oligonucleotides and / or PCR products. The fragment was cloned into pMK (kanR) using KpnI and SacI restriction sites. The plasmid DNA was purified (Pure Yield™ Plasmid Midiprep, Promega) from transformed bacteria and concentration determined by UV spectroscopy. The final construct was verified by sequencing. The sequence congruence within the used restriction sites was 100%.
example 2
Subcloning of the COS Luciferase Gene into the pCMV and pSV40 Vectors
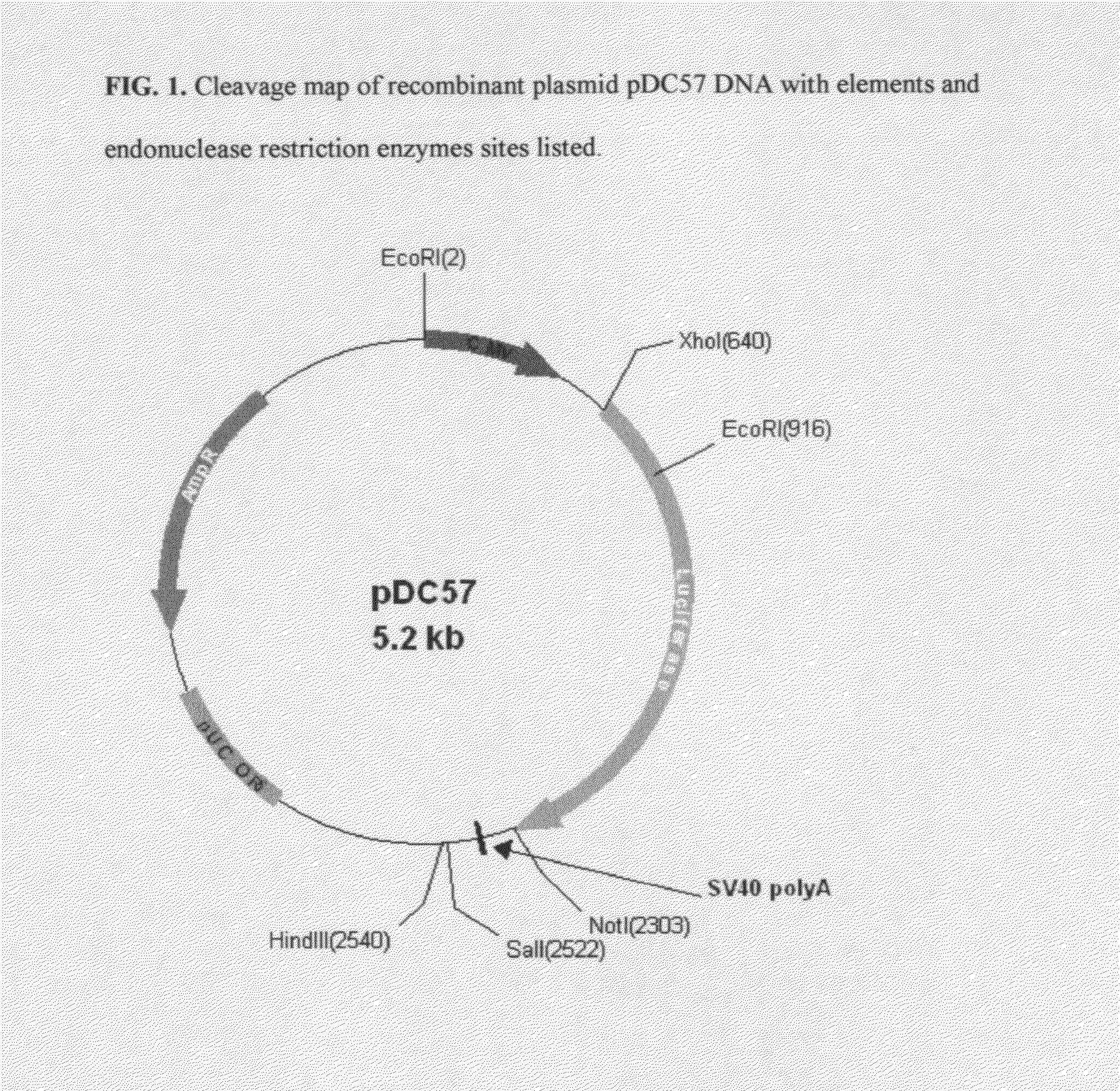
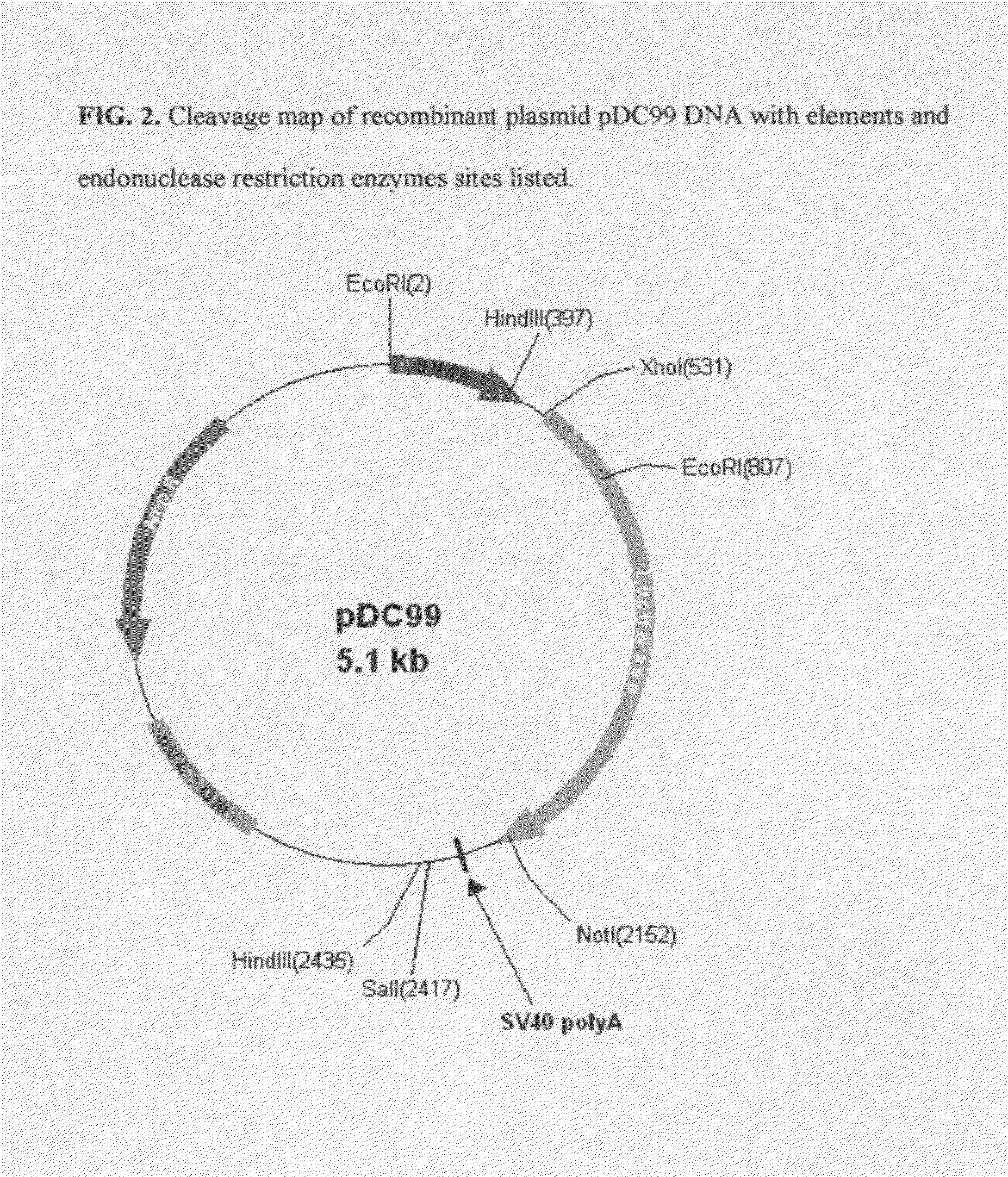
[0072]The synthetic COS luciferase assembled in Example 1 was excised from pMK cloning vector using flanking XhoI and NotI restriction enzymes (Fast Digest, Fermentas). The excised fragment was gel-purified (GenElute Gel Extraction Kit, Sigma) and quantitated using MassRuler™ DNA Ladder Mix (Fermentas). The excised gene was subcloned into both pCMV and pSV40 Mammalian Expression Vectors using corresponding XhoI and NotI restriction sites. The completed pCMV construct was named pDC57. The completed pSV40 construct was named pDC99.
example 3
Subcloning of the COS Luciferase Gene into the pNosdc Binary Vector for Expression in Plants
[0073]The synthetic COS luciferase assembled in Example 1 was amplified using the Polymerase Chain reaction. Amplification was performed with primers including XmaI and SacI restriction sites. The ends of the amplified fragment were cut with XmaI and SacI restriction enzymes (New England Biolabs) and the fragment was gel-purified (GenElute Gel Extraction Kit, Sigma) and quantitated using MassRuler™ DNA Ladder Mix (Fermentas). The amplified fragment was subcloned into the pNosdc binary vector for transformation of plants via Agrobacterium tumefaciens. The completed construct was named pNosdcCOS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com