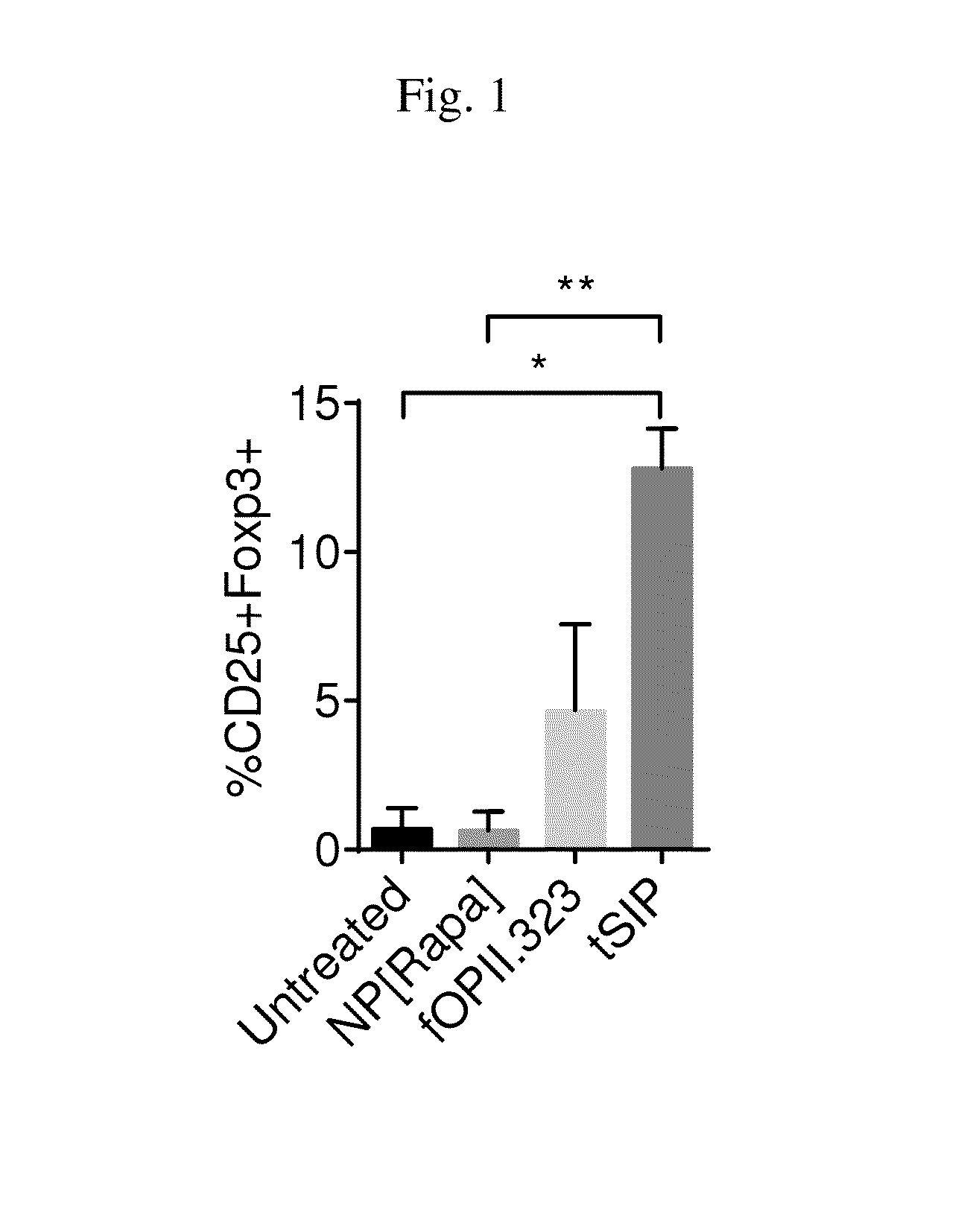
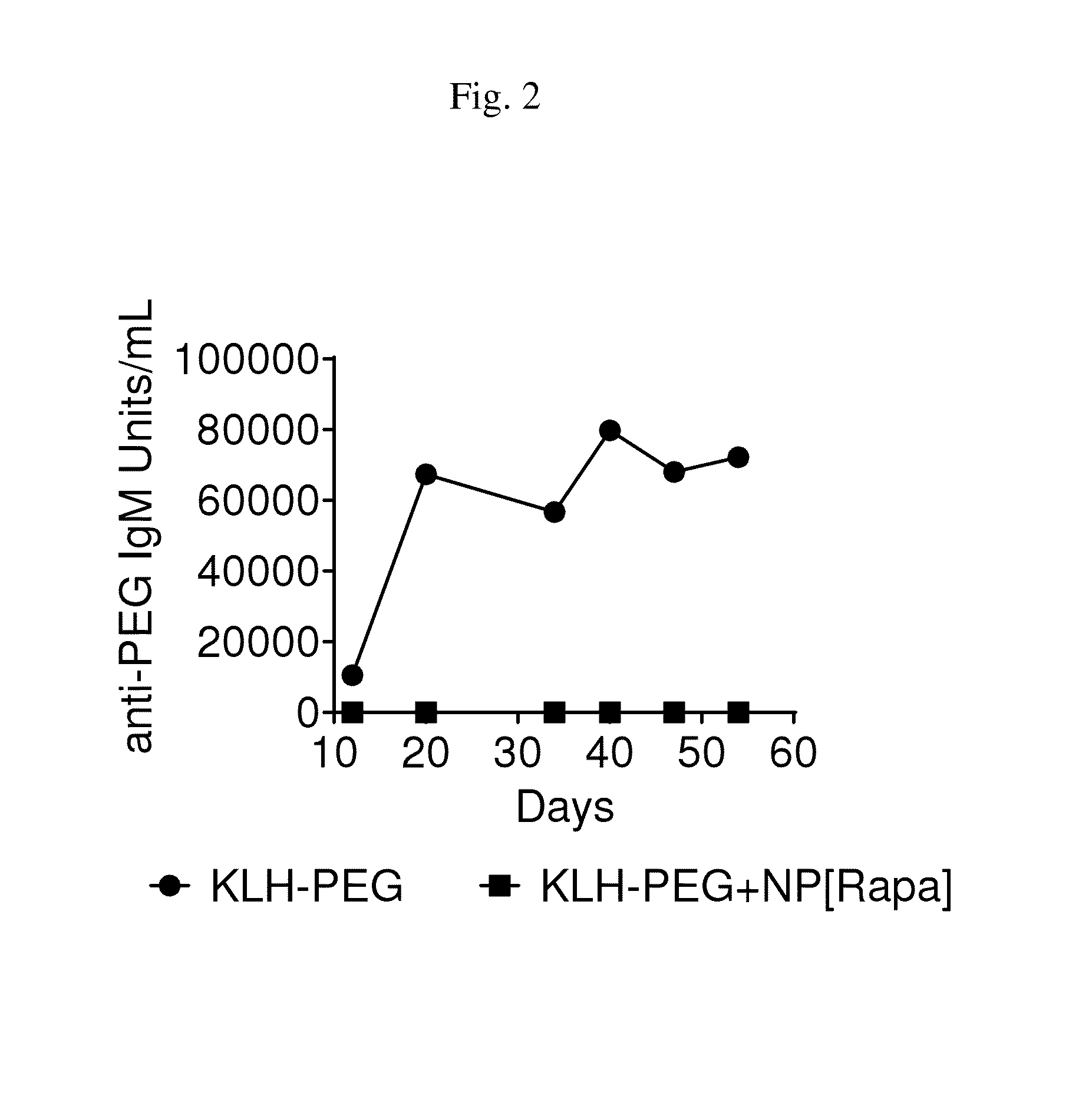
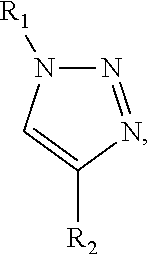
Methods and compositions for enhancing cd4+ regulatory t cells
a technology of regulatory t cells and immunosuppressants, applied in the direction of drug compositions, immunological disorders, metabolism disorders, etc., can solve the problems of overall systemic downregulation of the immune system, decrease of undesired immune responses, etc., and achieve the effect of enhancing the number or percentage (or ratio)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Polymeric Nanocarrier Containing Polymer-Rapamycin Conjugate (Prophetic)
Preparation of PLGA-Rapamycin Conjugate
[0186]PLGA polymer with acid end group (7525 DLG1A, acid number 0.46 mmol / g, Lakeshore Biomaterials; 5 g, 2.3 mmol, 1.0 eq) is dissolved in 30 mL of dichloromethane (DCM). N,N-Dicyclohexylcarbodimide (1.2 eq, 2.8 mmol, 0.57 g) is added followed by rapamycin (1.0 eq, 2.3 mmol, 2.1 g) and 4-dimethylaminopyridine (DMAP) (2.0 eq, 4.6 mmol, 0.56 g). The mixture is stirred at rt for 2 days. The mixture is then filtered to remove insoluble dicyclohexylurea. The filtrate is concentrated to ca. 10 mL in volume and added to 100 mL of isopropyl alcohol (IPA) to precipitate out the PLGA-rapamycin conjugate. The IPA layer is removed and the polymer is then washed with 50 mL of IPA and 50 mL of methyl t-butyl ether (MTBE). The polymer is then dried under vacuum at 35 C for 2 days to give PLGA-rapamycin as a white solid (ca. 6.5 g).
[0187]Nanocarrier containing PLGA-rapamycin is prepared a...
example 2
Preparation of Gold Nanocarriers (AuNCs) Containing Rapamycin (Prophetic)
Preparation of HS-PEG-Rapamycin
[0191]A solution of PEG acid disulfide (1.0 eq), rapamycin (2.0-2.5 eq), DCC (2.5 eq) and DMAP (3.0 eq) in dry DMF is stirred at rt overnight. The insoluble dicyclohexylurea is removed by filtration and the filtrate is added to isopropyl alcohol (IPA) to precipitate out the PEG-disulfide-di-rapamycin ester and washed with IPA and dried. The polymer is then treated with tris(2-carboxyethyl)phosphine hydrochloride in DMF to reduce the PEG disulfide to thiol PEG rapamycin ester (HS-PEG-rapamycin). The resulting polymer is recovered by precipitation from IPA and dried as previously described and analyzed by H NMR and GPC.
[0192]Formation of Gold NCs (AuNCs):
[0193]An aq. solution of 500 mL of 1 mM HAuC14 is heated to reflux for 10 min with vigorous stirring in a 1 L round-bottom flask equipped with a condenser. A solution of 50 mL of 40 mM of trisodium citrate is then rapidly added to t...
example 3
Mesoporous Silica Nanoparticles with Attached Ibuprofen (Prophetic)
[0196]Mesoporous SiO2 nanoparticle cores are created through a sol-gel process. Hexadecyltrimethyl-ammonium bromide (CTAB) (0.5 g) is dissolved in deionized water (500 mL), and then 2 M aqueous NaOH solution (3.5 mL) is added to the CTAB solution. The solution is stirred for 30 min, and then Tetraethoxysilane (TEOS) (2.5 mL) is added to the solution. The resulting gel is stirred for 3 h at a temperature of 80° C. The white precipitate which forms is captured by filtration, followed by washing with deionized water and drying at room temperature. The remaining surfactant is then extracted from the particles by suspension in an ethanolic solution of HCl overnight. The particles are washed with ethanol, centrifuged, and redispersed under ultrasonication. This wash procedure is repeated two additional times.
[0197]The SiO2 nanoparticles are then functionalized with amino groups using (3-aminopropyl)-triethoxysilane (APTMS)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com