Anode for oxygen generation and manufacturing method for the same
an oxygen generation and anode technology, applied in the direction of manufacturing tools, electrical-based machining electrodes, electrode coatings, etc., can solve the problems of increasing the current of the electrolysis product, the increase of the electrolysis voltage after electrolysis, and the inability to light the electrolysis product, so as to suppress the crystallite diameter of iridium oxide, and reduce the overvoltage of the oxygen generation electrode
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0098]The surface of titanium plate (JIS-I) was subjected to the dry blast with iron grit (G120 size), followed by pickling in an aqueous solution of concentrated hydrochloric acid for 10 minutes at the boiling point for cleaning treatment of the metal substrate of the electrode. The cleaned metal substrate of the electrode is set to the AIP unit applying Ti—Ta alloy target as a vapor source and a coating of tantalum and titanium alloy was applied as the AIP base layer on the surface of the metal substrate of the electrode. Coating condition is shown in Table 1.
[0099]The coated metal substrate was treated at 530° C. in an electric furnace of air circulation type for 180 minutes.
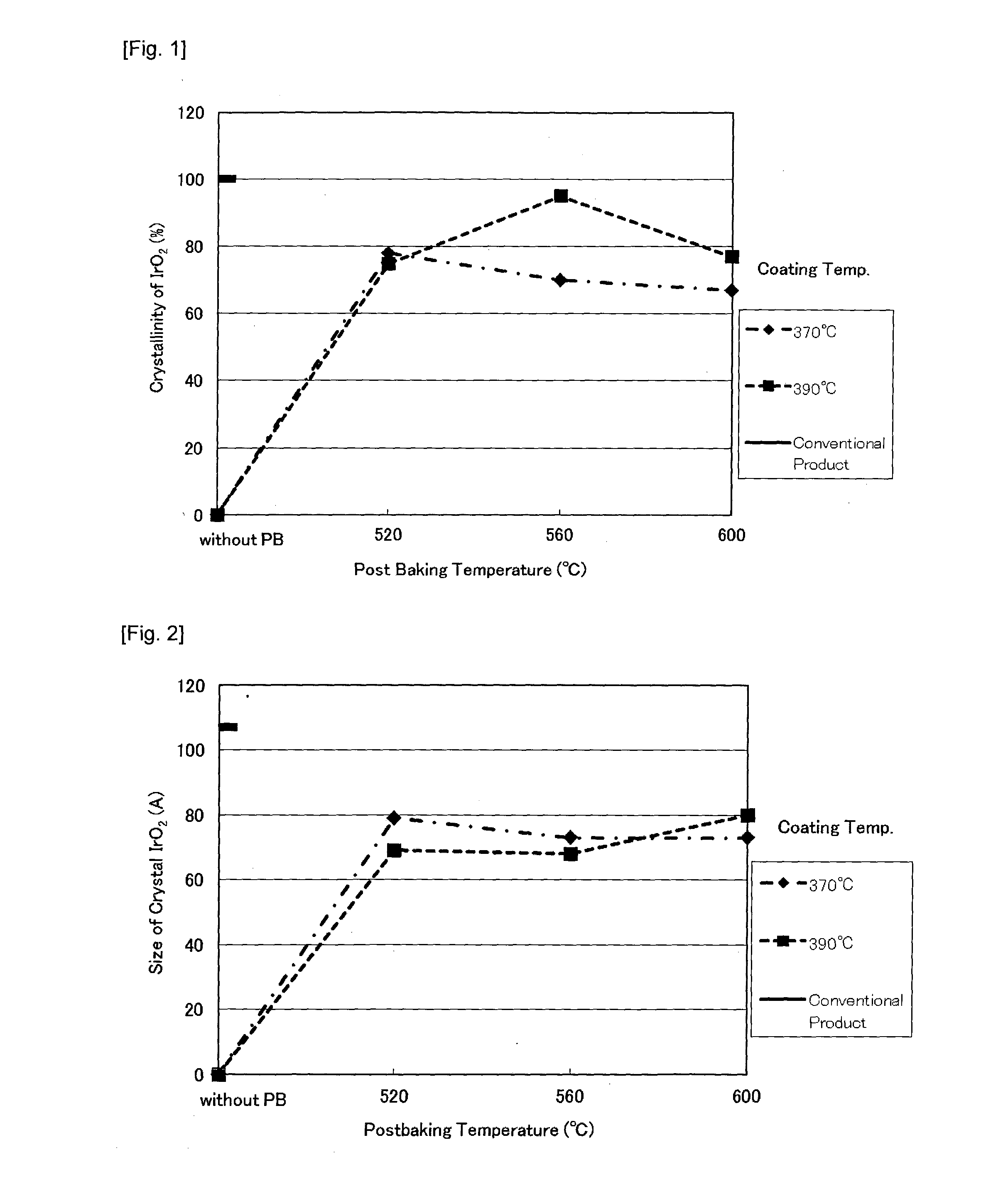
[0100]Then, the coating solution prepared by dissolving iridium tetrachloride and tantalum pentachloride in concentrated hydrochloric acid is applied on the coated metal substrate. After drying, the thermolysis coating was conducted for 15 minutes in the electric furnace of air circulation type at 370° C. to ...
example 2
[0105]The electrode for evaluation was manufactured in the same manner as with Example 1 except that post-bake was conducted in an electric furnace of air circulation type for one hour at 560° C. and the same electrolysis evaluation was performed.
[0106]The X-ray diffraction performed after post-bake showed the degree of crystallinity and crystallite diameter of IrO2 in the catalyst layer equivalent to Example 1.
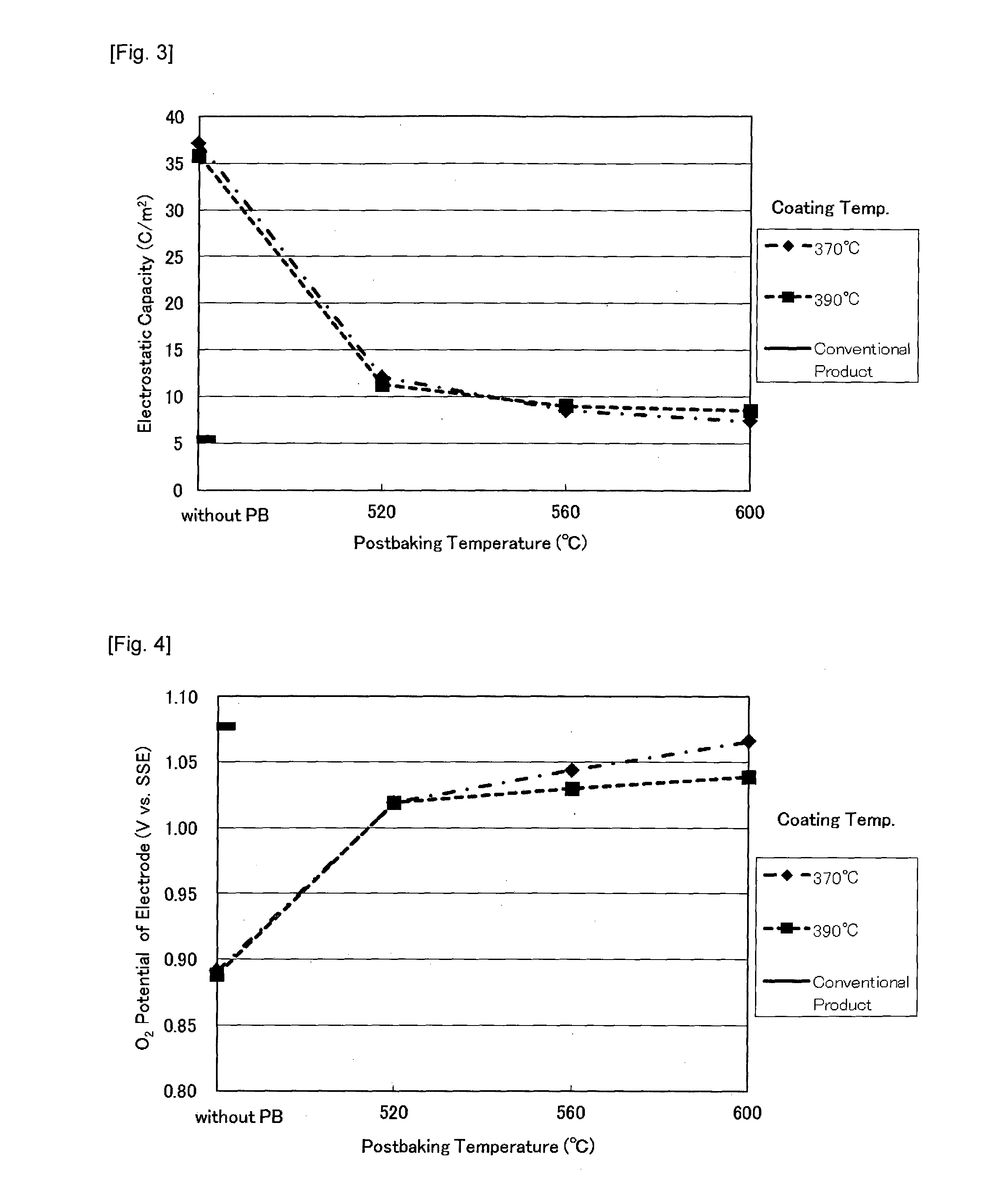
[0107]As shown in Table 3, an amount of a lead adhesion to the electrode of Example 2 is one-fourth to that of the Comparative Example 1 and a suppression effect of the lead adhesion was confirmed. In addition, the accelerated electrolysis life was increased to 80% and their durability has also have been improved.
example 3
[0108]The electrode for evaluation was manufactured in the same manner as with Example 1 except that post-bake was conducted in an electric furnace of air circulation type for one hour at 600° C. and the same electrolysis evaluation was performed.
[0109]The X-ray diffraction performed after post-bake showed the degree of crystallinity and crystallite diameter of IrO2 in the catalyst layer equivalent to Example 1.
[0110]As a result of an electrolysis evaluation, as shown in Table 3, an amount of a lead adhesion and an electrolysis life was equivalent to Example 2 and a suppression effect of the lead adhesion was confirmed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com