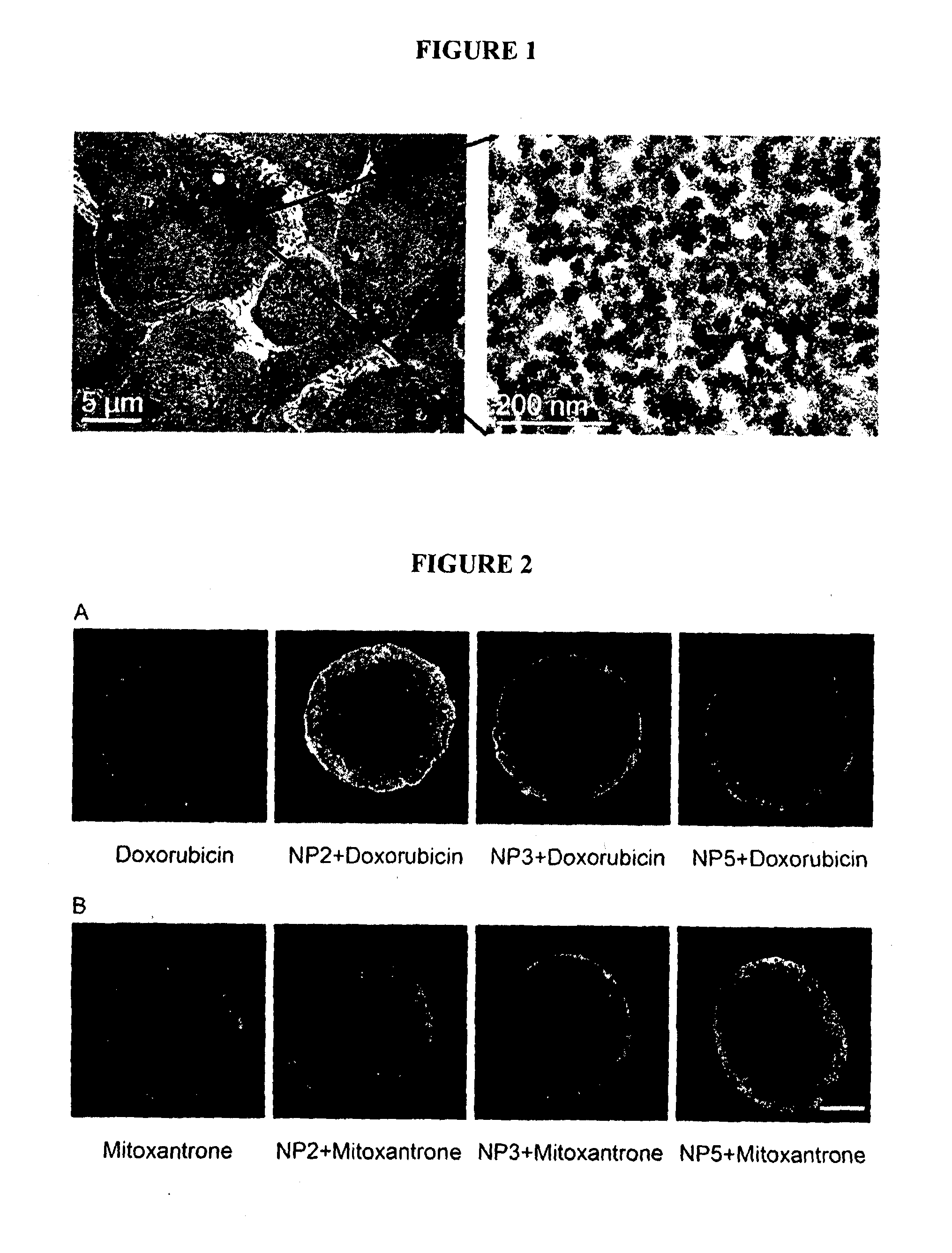
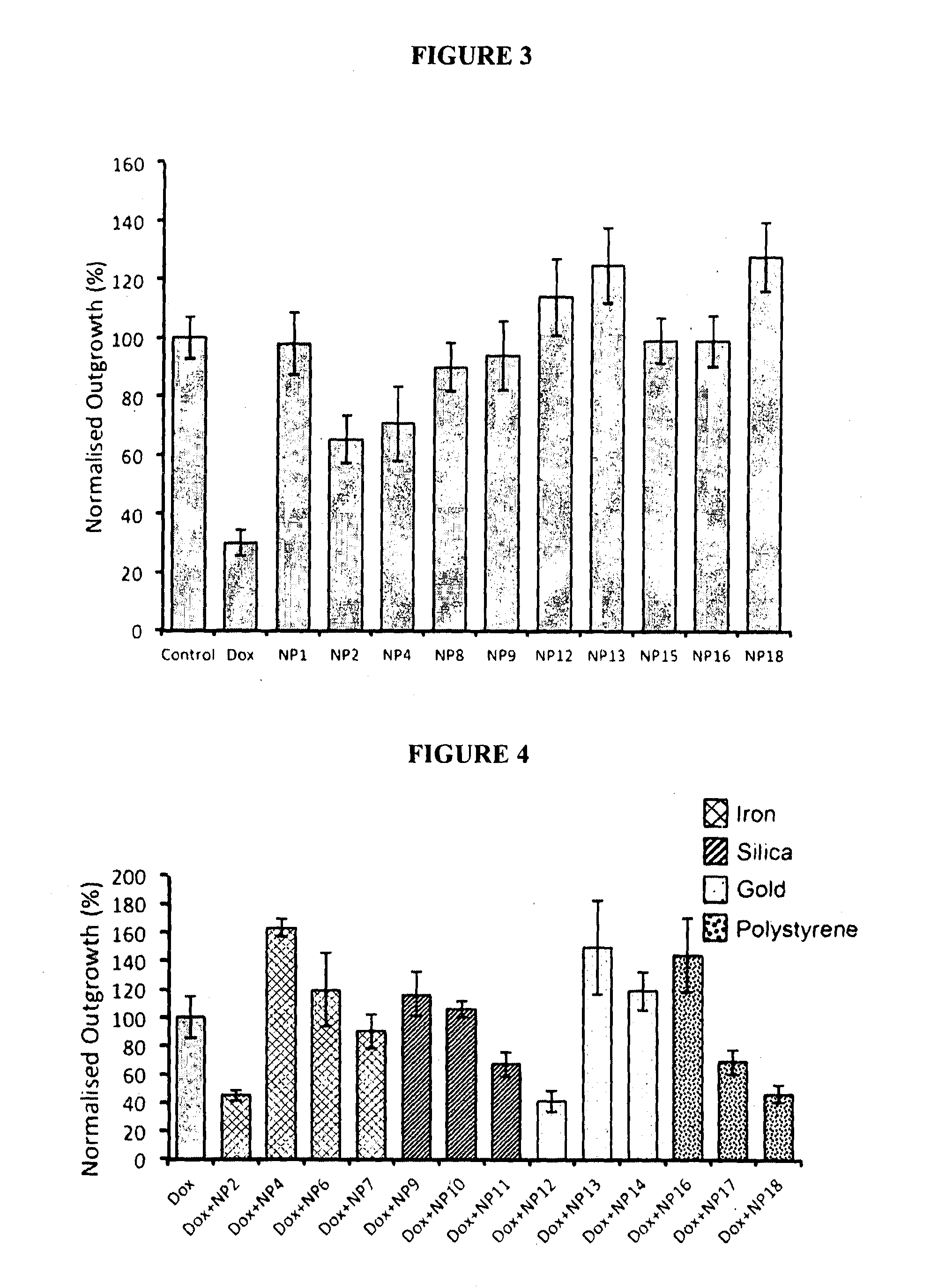
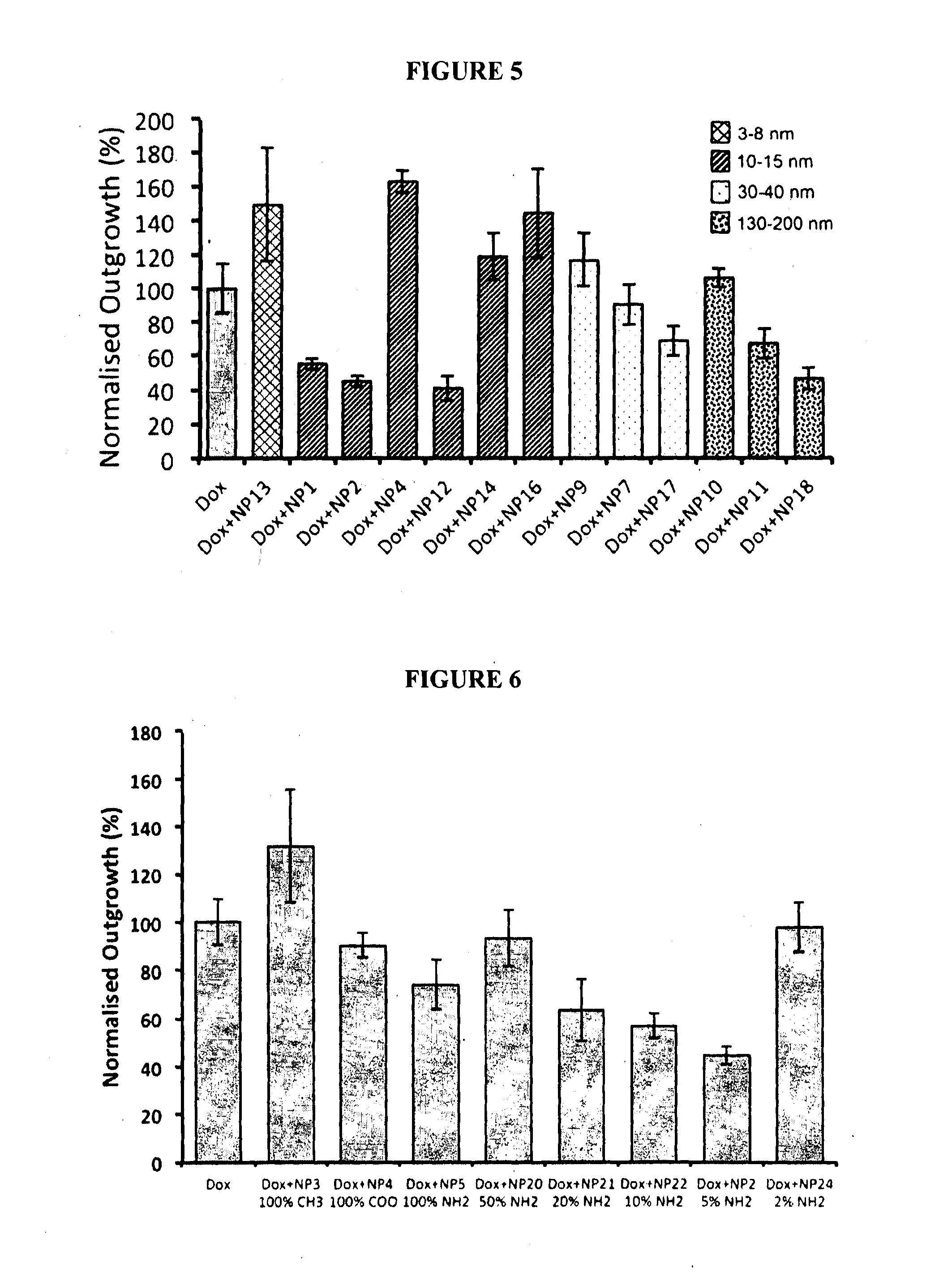
Method for the Treatment of a Solid Tumour
a solid tumour and treatment method technology, applied in the field of neoplastic conditions, can solve the problems of unsustainable immunological approaches to the treatment of cancer for over a century, severe side effects, and cancer is likely to become the most common fatal disease in these countries, and achieve the effect of specific targeting of the tumour
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Steric Stabilization of Iron Oxide Nanoparticles in Aqueous Dispersion Using poly(monoacryloxyethyl phosphate)10-block-poly(acrylamide)20 Macro Raft Agent
Part (a): Preparation of Diluted Aqueous Ferrofluid Stable in Acidic Medium
[0276]Magnetite nanoparticles were produced following the method of Massart (Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Transactions on Magnetics, 1981. MAG-17(2): p. 1247-1248). In a typical reaction, 80 ml of 1M FeCl3.6H2O in 2M HCl and 40 ml of 1M FeCl2.4H2O in 2M HCl were mixed in a 2 Litre beaker and the mixture diluted to 1.2 Litre with MQ-water. 250 ml of NH4OH (28% (w / w)) was then quickly added to the beaker and the mixture vigorously stirred for 30 minutes. Upon adding NH4OH, the colour of the mixture immediately turned from orange to black suggesting the formation of magnetite. Magnetite was then oxidized in acidic medium to maghemite by heating at 90° C. with iron nitrate for about an hour. The colour of the suspens...
example 2
Steric Stabilization of Iron Oxide Nanoparticles in Aqueous Dispersion Using 95% poly(monoacryloxyethyl phosphate)10-block-poly(ethylene oxide)17 Macro Raft Agent and 5% poly(monoacryloxyethyl phosphate)10-block-poly(acrylamide)25 Macro Raft Agent
Part (a): Esterification of poly(ethylene glycol)monomethyl ether with 2-{[(butylsulfanyl)carbonothioyl]sulfanyl}propanoic Acid
[0279]MethoxyPEG (Mn ˜798) was warmed and stirred to liquefy and homogenize it, and 19.95 g (25.0 mmol) was then weighed into a 250 mL 3-necked round bottom flask, and then allowed to solidify. 2-{[(butylsulfanyl)carbonothioyl]sulfanyl}propanoic acid (6.96 g, 29.3 mmol) and 4-dimethylaminopyridine (360 mg, 2.9 mmol) were added to the flask, a magnetic stirbar was introduced, and the flask was purged with nitrogen. Dry dichloromethane (75 mL) was added and the mixture was stirred until the solids had all dissolved. The flask was then cooled in an ice bath and a solution of N,N′-dicyclohexylcarbodiimide (6.03 g, 29.3 ...
example 3
Steric Stabilization of Iron Oxide Nanoparticles in Aqueous Dispersion Using the Ferrofluid of Example 1 Part (a) and the poly(monoacryloxyethyl phosphate)10-block-poly(ethylene oxide)17 Macro Raft Agent of Example 2 Part (b)
[0284]Nanoparticle dispersion (8.0 g) prepared according to example 1 part (a) was diluted with 50 g of MQ water to yield a 0.5 wt % dispersion of the nanoparticles. The pH of this prepared nanoparticle dispersion was then raised to 5. The 0.5 wt % dispersion of iron oxide maintained at the same pH was then added to the 50 g of macro-RAFT agent from example 2 part (b). The mixture was vigorously stirred for 2 hours at room temperature before the pH was adjusted to 7.0. The mixture was then left stirring for another 3 hours. At this pH the copolymer remained partially neutralized while the nanoparticles were sufficiently above their point of zero charge to also be stable. The dispersion was then dialysed to remove salts, residual solvents, unwanted low molecular ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com