Methods of measuring potential for therapeutic potency and defining dosages for autologous cell therapies
a technology of autologous cells and potency, applied in the field of measuring potential for therapeutic potency and defining dosages of autologous cells, can solve the problems of significant and expensive clinical care decisions, increase the cost of these proposed therapeutic candidates, and significant labor and complex reagents and materials costs of manipulations, so as to reduce the potential contamination of the therapeutic tissues, reduce the cost, and enhance the effect of safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
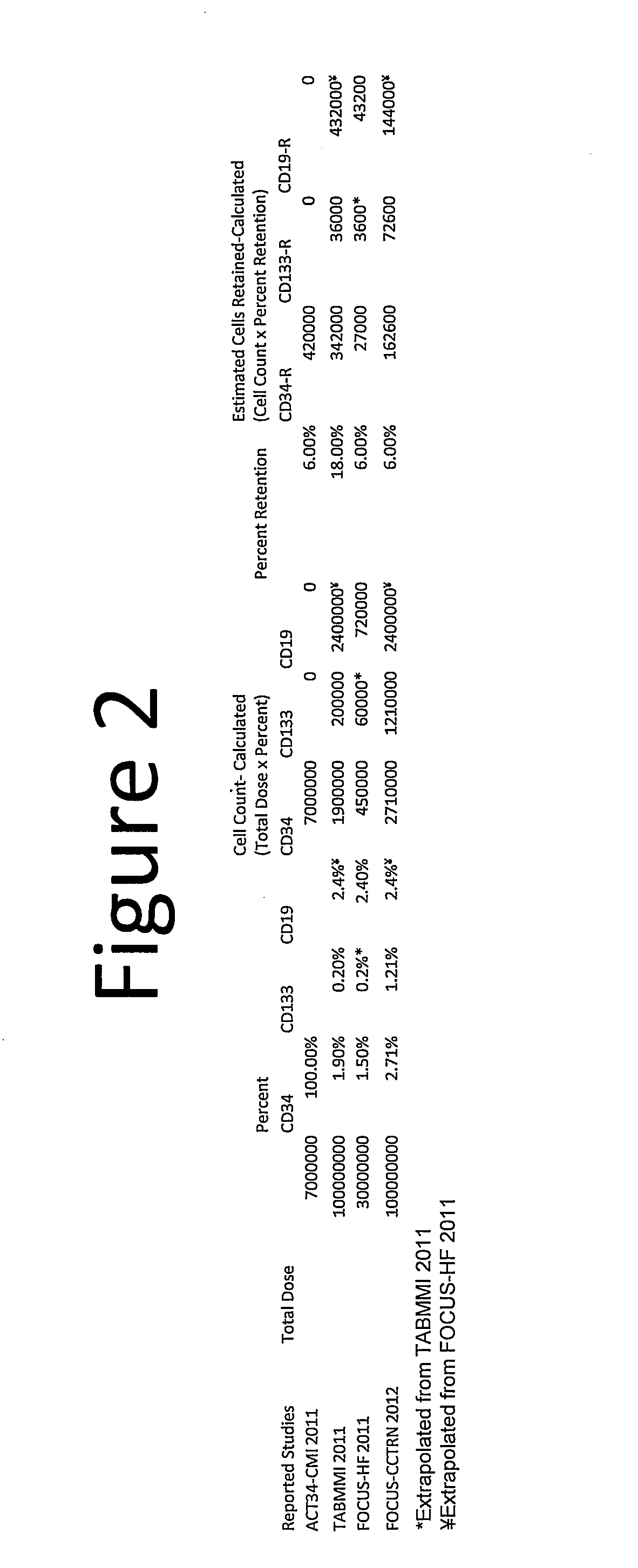
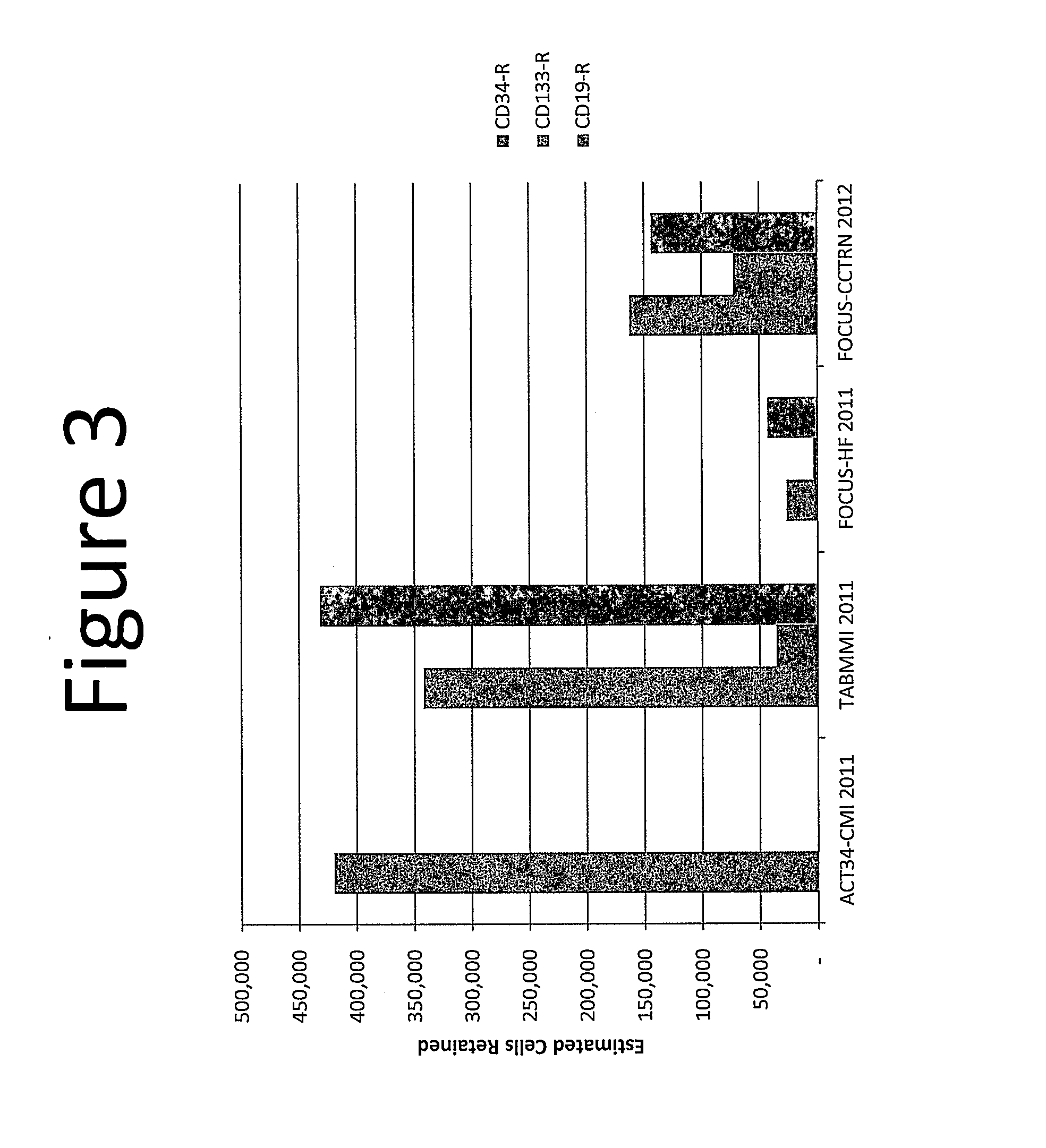
[0051]This Example describes the selection of patients for autologous tissue therapies based on the analysis of small tissue samples from the patients in advance of the therapy. Such tissue samples and patient selection is determined based on pre-defined thresholds of cellular characteristics such as number of cells per unit volume of tissue, functional capacities, gene expression profiling, and / or cell surface markers such as the levels of known cluster of differentiation (CD) surface markers of the constituent cells. The use of CD markers for immunophenotyping is well known in the art and described in commonly available resources such as: http: / / en.wikipedia.org. / wiki / Cluster_of_differentiation. CD thresholds selected are dependent on the disease being treated and are determined based on past studies where patient improvement has been correlated with the levels of certain CD cell surface markers or other biomarkers in their tissue. In the case of heart failure patients of ischemic...
example 2
[0052]Here we calculate the thresholds for a cardiac therapy as in Example 1. In Example 2, the assumptions are changed to use the effective dosage of at least 162,600 CD34 cells from the FOCUS-CCTRN clinical trial which uses autologous bone marrow mononuclear cells isolated using density gradient centrifugation with Ficoll Paque, instead of the estimated CD34 cells from the ACT34-CMI trial. The same assumption of no cell losses due to cell processing was applied. For our purpose, any reagent such as lymphocyte separating medium having the same characteristics and density as Ficoll Paque could also be used in the cell processing. Therefore, assuming that a full 60 ml of bone marrow aspirate is processed from the patient, to achieve the effective dosage of at least 162,600 CD34 cells, and / or 72,000 CD133 cells and / or 144,000 CD19 cells using the Helix transendocardial delivery system, the minimum number of cells needed to be present per ml of bone marrow aspirate harvested from the p...
example 3
[0053]In Example 3, instead of isolating autologous bone marrow mononuclear cells using density gradient centrifugation with Ficoll Paque or other similar reagent, we use a point of care cell processing system which concentrates autologous bone marrow or blood-derived mononuclear cells using the same technique of density gradient centrifugation without the addition of Ficoll Paque. In this case, we further assume 20 to 50 percent cell losses due to the cell processing. Therefore, assuming that 54 ml of bone marrow aspirate is processed from the patient (it should be noted that 54 ml of bone marrow aspirate is used here to take into account the 6 ml of heparin or other anticoagulating agent used to typically make up the full volume of 60 ml during bone marrow harvest), to achieve the same effective dosage of at least 162,600 CD34 cells from the FOCUS-CCTRN clinical trial, and / or 72,600 CD133 cells, and / or 144,000 CD19 cells using the Helix transendocardial delivery system, the minimu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com