Compositions and methods for treating microbial infections
a technology for microbial infections and compositions, applied in the field of treatment of microbial infections, can solve the problems of preventing the identification of small molecules that target pathways, unable to identify candidate therapeutics, and a huge threat,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
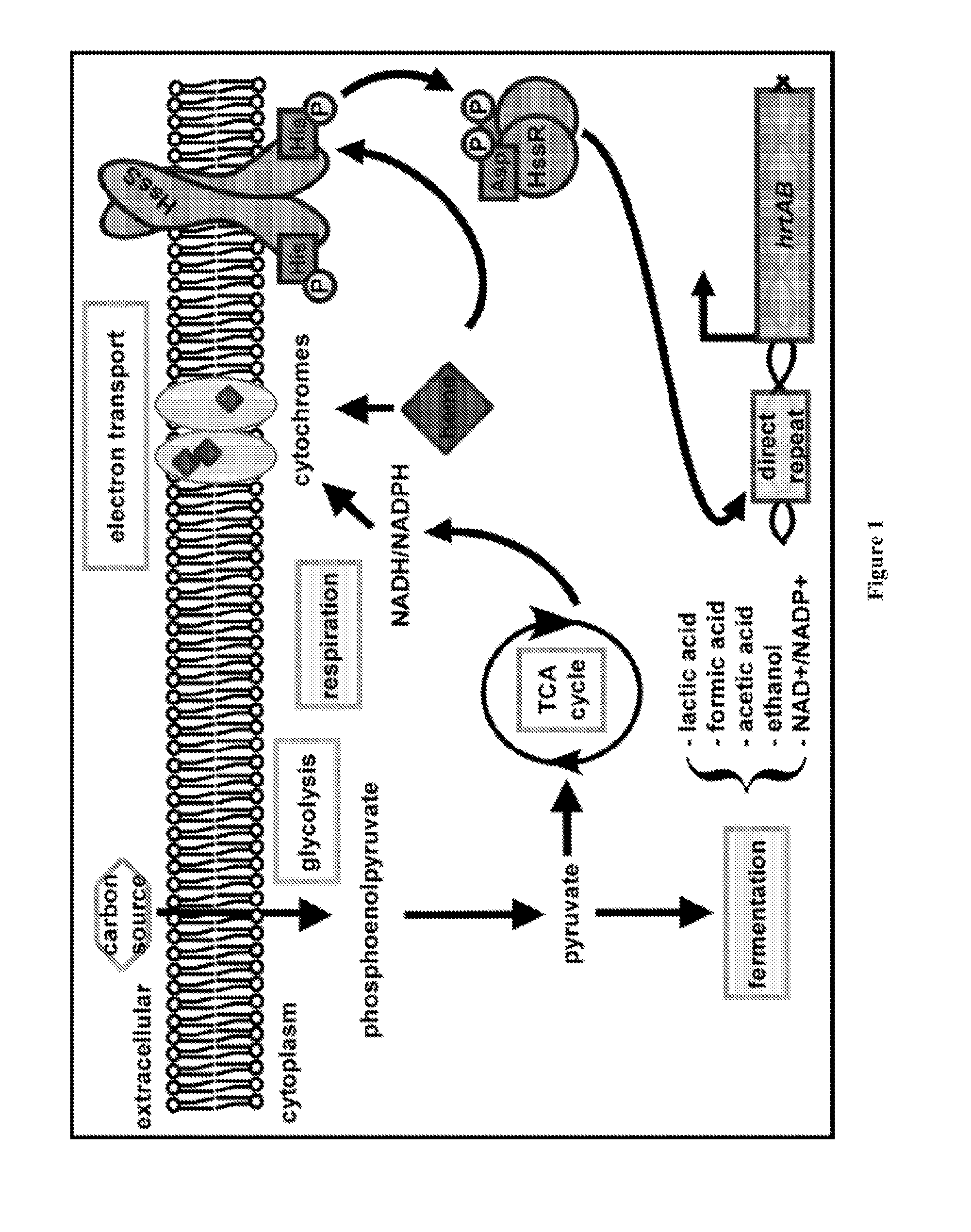
[0106]Like most bacteria, S. aureus requires iron to successfully infect its host. Iron is a cofactor for essential enzymes involved in host defense, DNA replication, and energy production. In humans most iron is found in the form of heme. S. aureus preferentially uses host heme as an iron source during infection (72). It does this using the iron-regulated surface determinant (Isd) system to import heme into the cell (79). In the cytoplasm heme can either be used intact as a cofactor or degraded to release free iron. In addition to stealing host heme, S. aureus can synthesize heme de novo.
[0107]Despite being an essential part of staphylococcal biology, heme is toxic. With reference to FIG. 1, bacterial gene regulation is controlled by a number of signal transduction systems known as two-component regulatory systems (TCS) (1-7). The present inventors identified a novel TCS in many Gram positive pathogens that responds to alterations in available heme, a critical cofactor of bacterial...
example 2
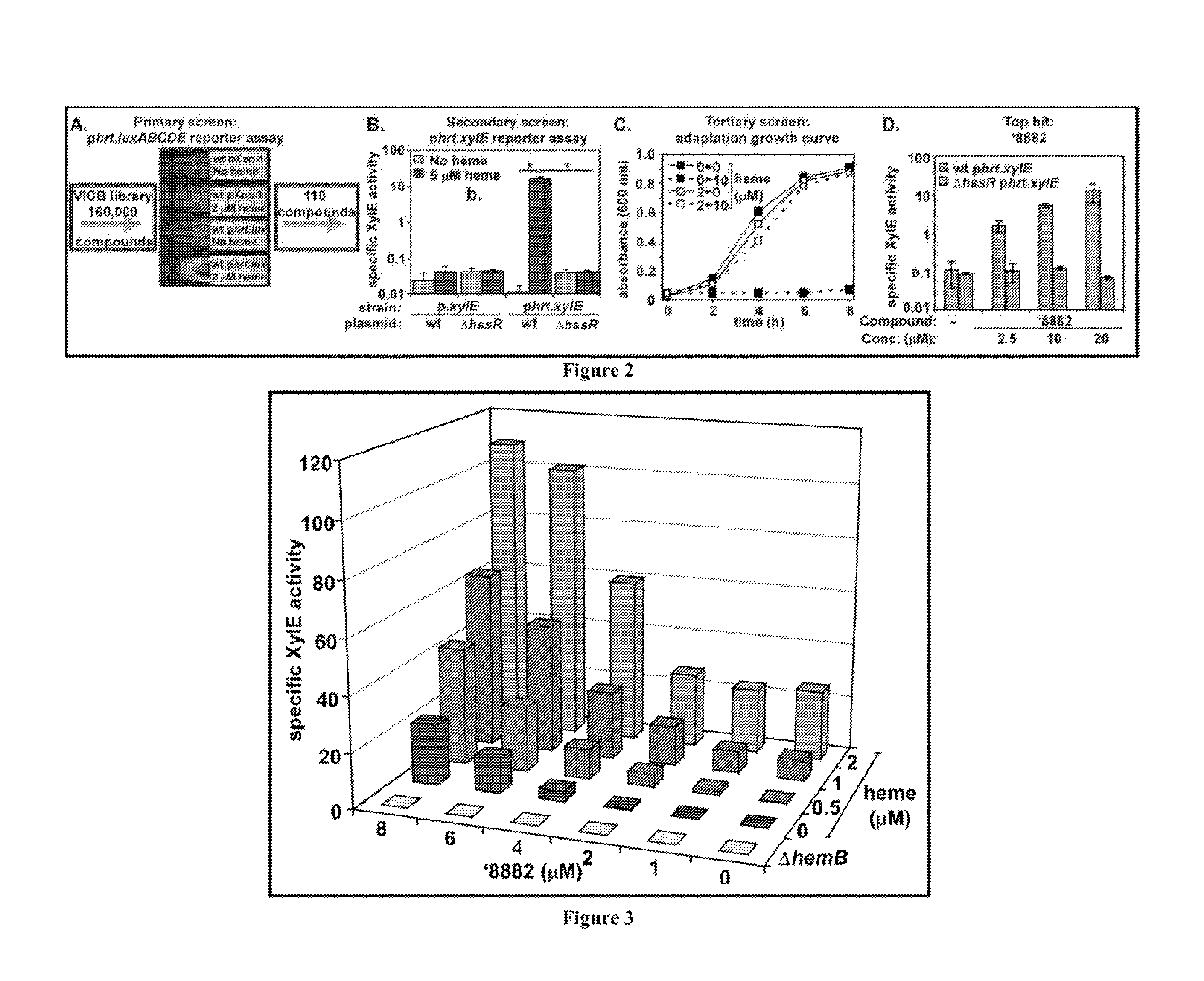
[0122]The structure, purity, and heme-activating properties of '882 were verified following its resynthesis by way of a five-step reaction sequence starting from commercially available 1-hydroxy-2-naphthaldehyde (44).
Synthesis and Relative HssRS Activation of Analogues '8882(1)
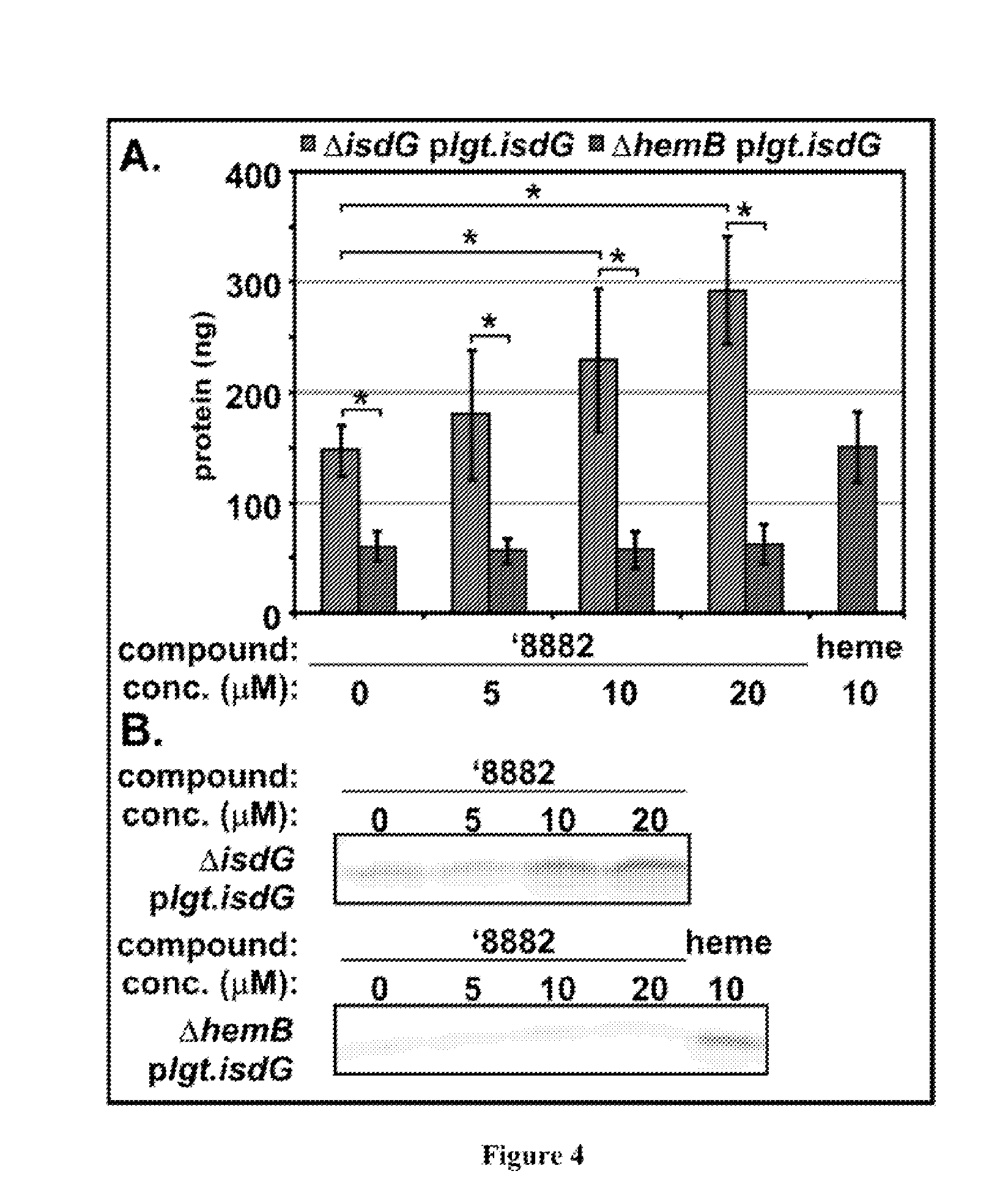
[0123]Both purchased and resynthesized '882 equally maintain HssRS activation. Moreover, the free base of '882 and its corresponding trifluoroacetate salt demonstrate good chemical stability, have no rule of five violations, and exhibit good solubility in DMSO and aqueous solution (45). Using an iterative medicinal chemistry approach, preliminary structure activity relationship (SAR) data has been collected (summarized above). The identification of positions around the '882 scaffold that tolerate structural modification is useful in the development of an affinity-probe derived from '882.
[0124]Analogs of '882 were divided into two classes, and chemical modifications of both the Western-naphthol ring and the Eas...
example 3
[0126](Prophetic, in part)—Sensor probe based on an '882 scaffold.
[0127]Based on the SAR data of '882, the present inventors propose the design of chemical probes for the purpose of target identification. Probes are prepared and examined in the context of affinity chromatography and photoaffinty labeling strategies (48-50). The former approach will rely on streptavidin chromatography in conjunction with immobilization of the biotin conjugate 5. Boc-protected pyrazole 3 have been prepared and it has been determined that 3 activates HssRS as measured by XylE assay. The present inventors are now working toward PEG-linked Boc-protected amine 4. Treatment of 4 with trifluoroacetic acid, followed by condensation with biotin will afford conjugate 5 ready for affinity chromatography experiments.
[0128]In addition to affinity chromatography a series of photoaffinity probes will be designed, prepared, and evaluated (51). Key elements of photoaffinity ligand (PAL) design are selection of a phot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
| Antimicrobial properties | aaaaa | aaaaa |
| Environmental properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com