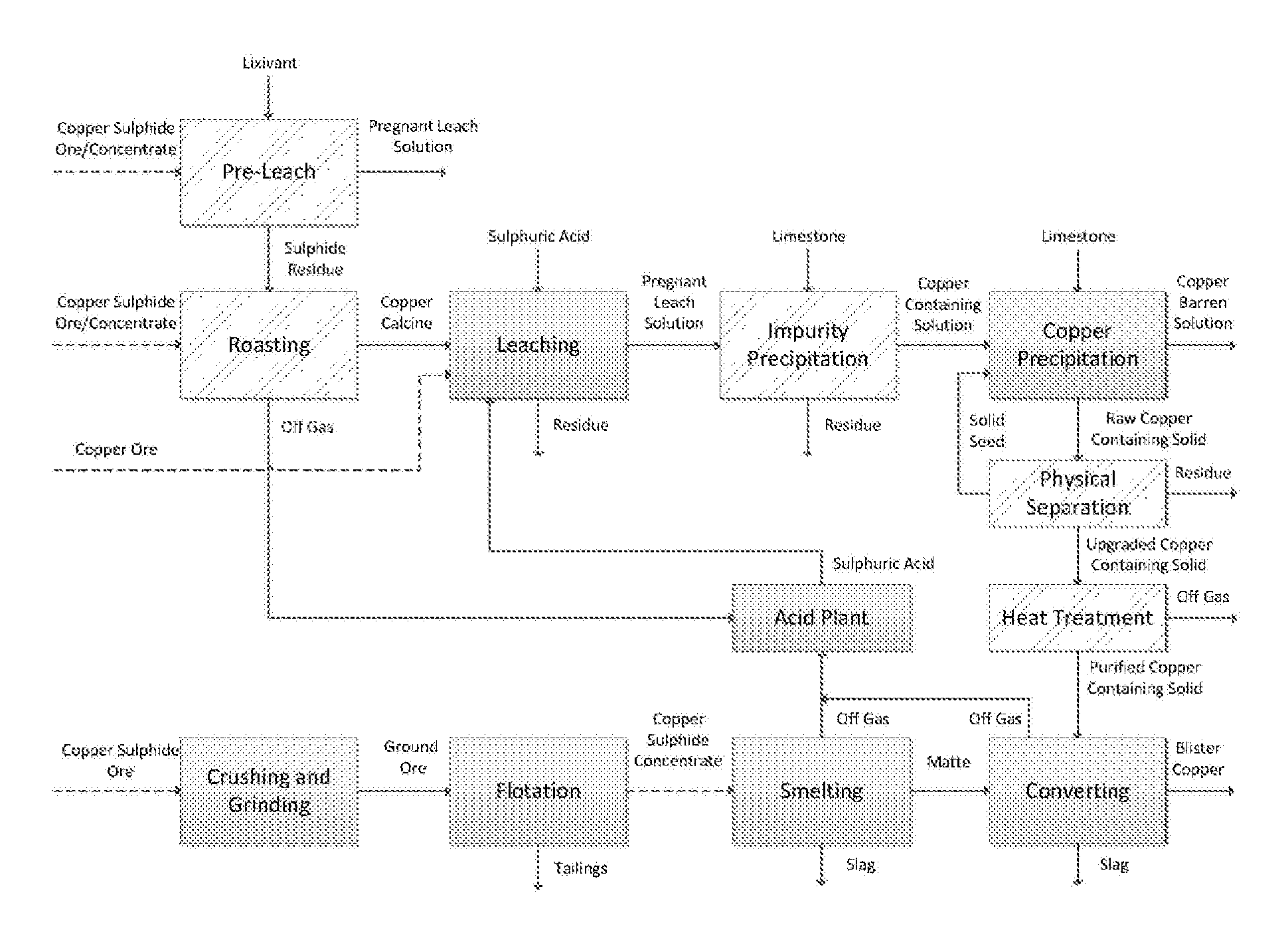
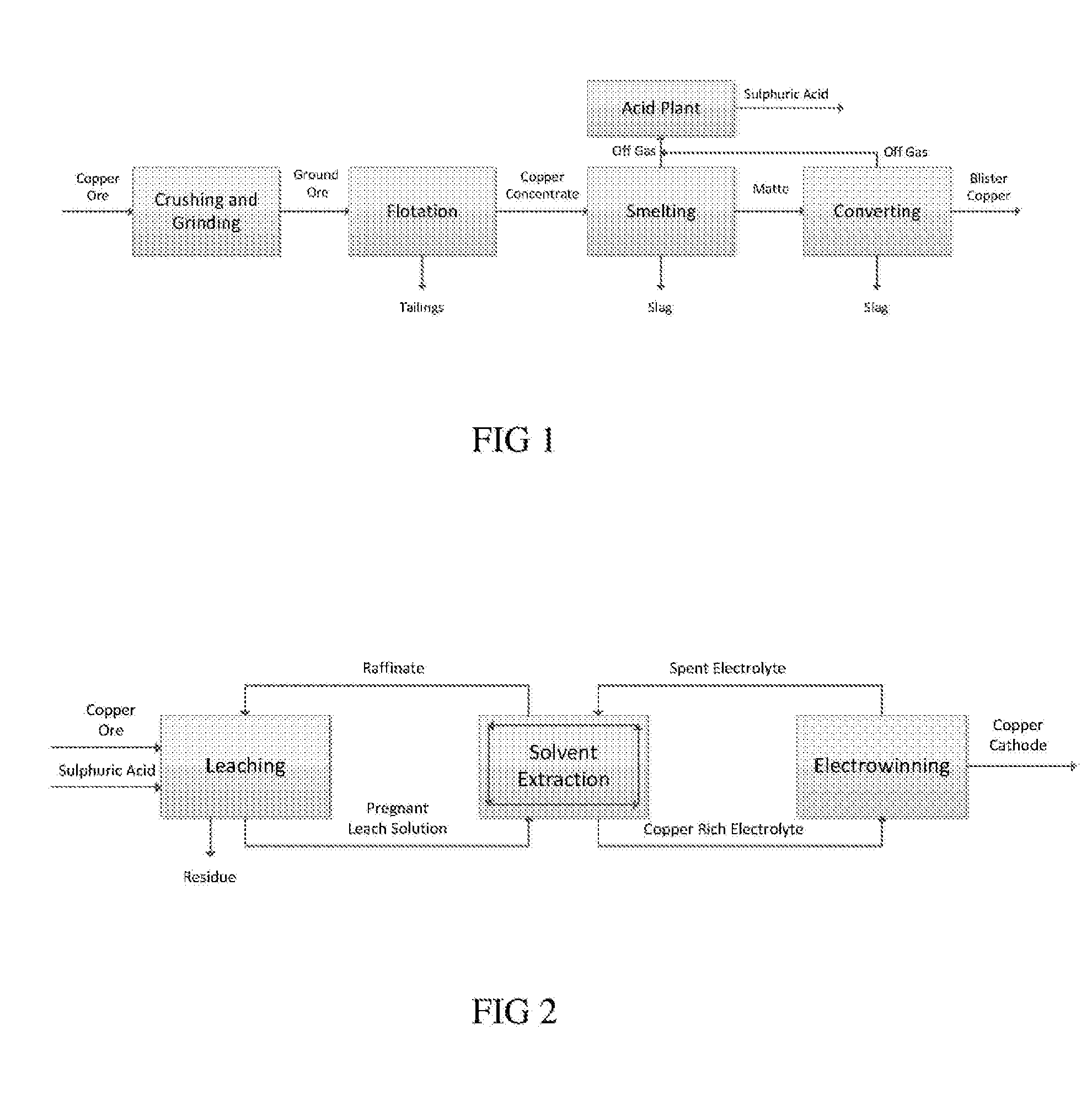
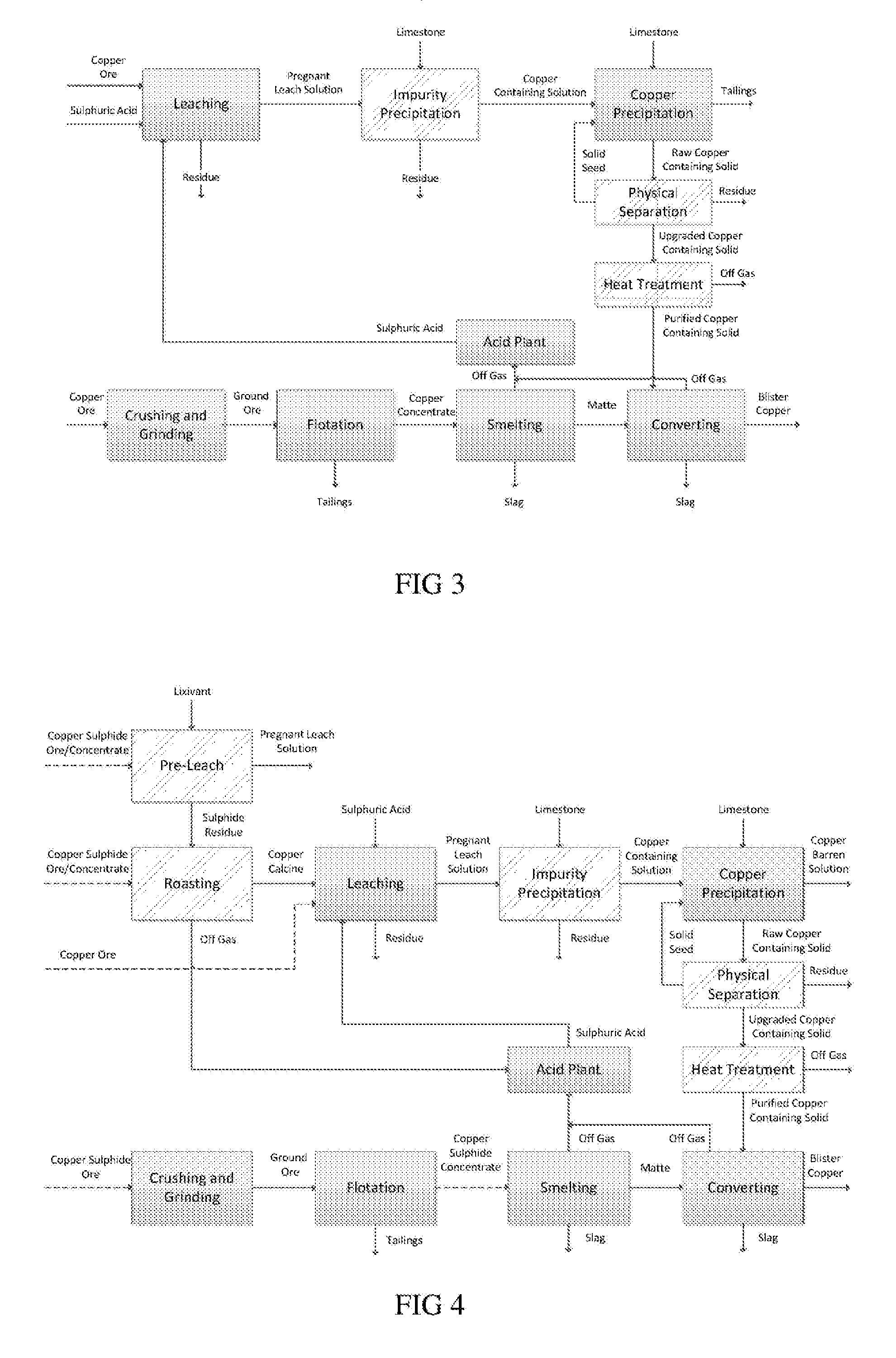
Copper processing method
a processing method and copper technology, applied in the field of copper processing method, can solve the problems of difficult control of the heat balance in these process units, difficult identification of new high-grade ore deposits, and deeper disadvantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Oxidative Roast
[0138]A copper concentrate was produced from a copper sulphide ore using, a lab scale flotation cell. The concentrate contained mostly chalcopyrite with a small amount of silica and pyrite. Separate samples of the concentrate were heated to 600° C., 750° C. and 900° C. in a tube furnace. An atmosphere of sulphur dioxide and air was enforced. The ratio of the sulphur dioxide and to air and therefore oxygen in the furnace was controlled by adjusting, the flow rate of these gases into the furnace. A single flow rate set point for each gas was chosen based on Factsage modelling. All three experiments were carried out at the same flow rates and therefore the same atmospheric conditions. The flow conditions were 400 mL-air / min and 25 mL-SO2 / min which equates to an enforced atmosphere of about 0.06 atm SO2, 0.94 atm air which is equivalent to 0.20 atm O2. All solids formed were characterised by Powder XRD. All solids contained some silica.
TABLE 1Products of oxidative roastTe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com