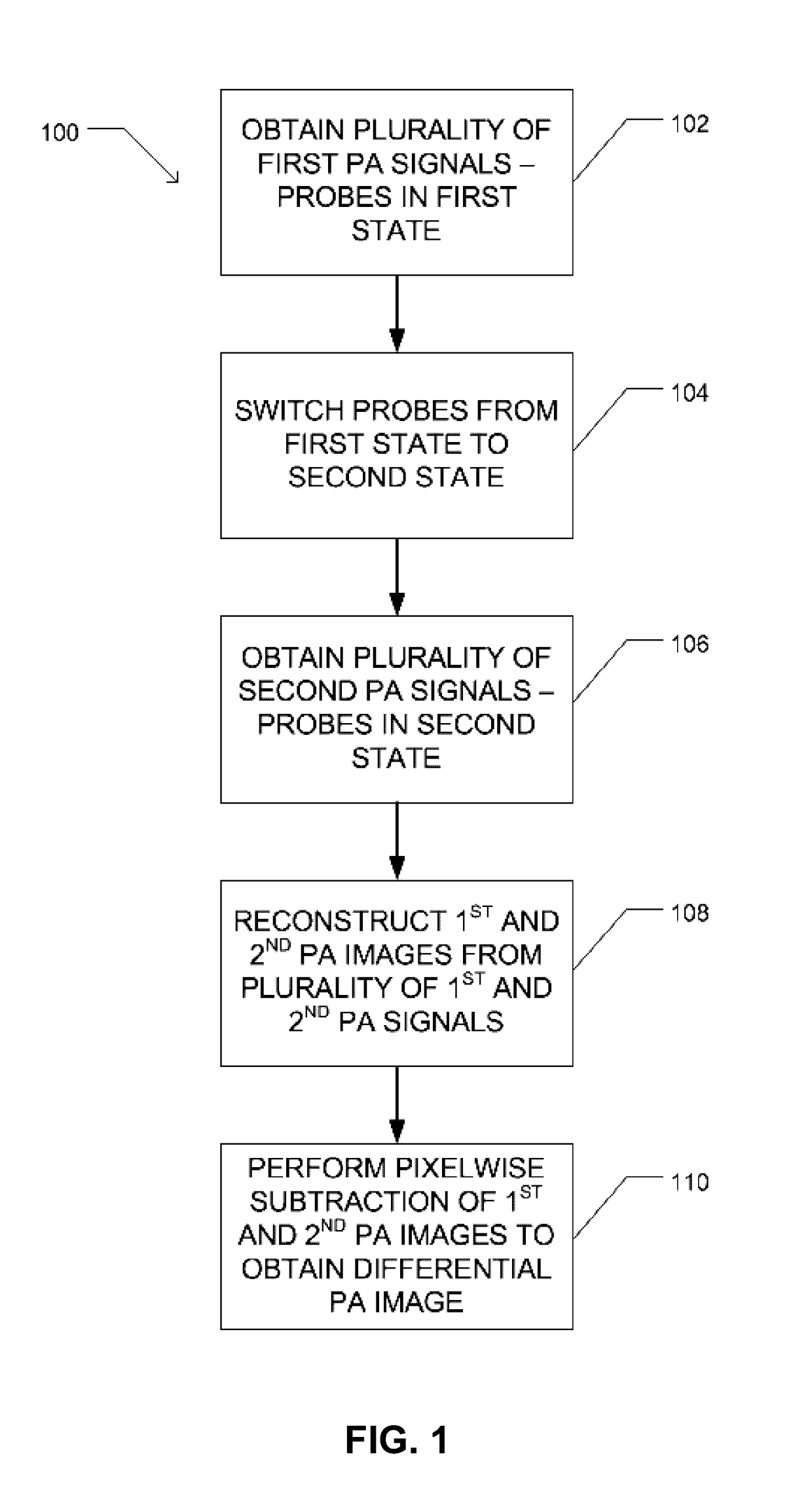
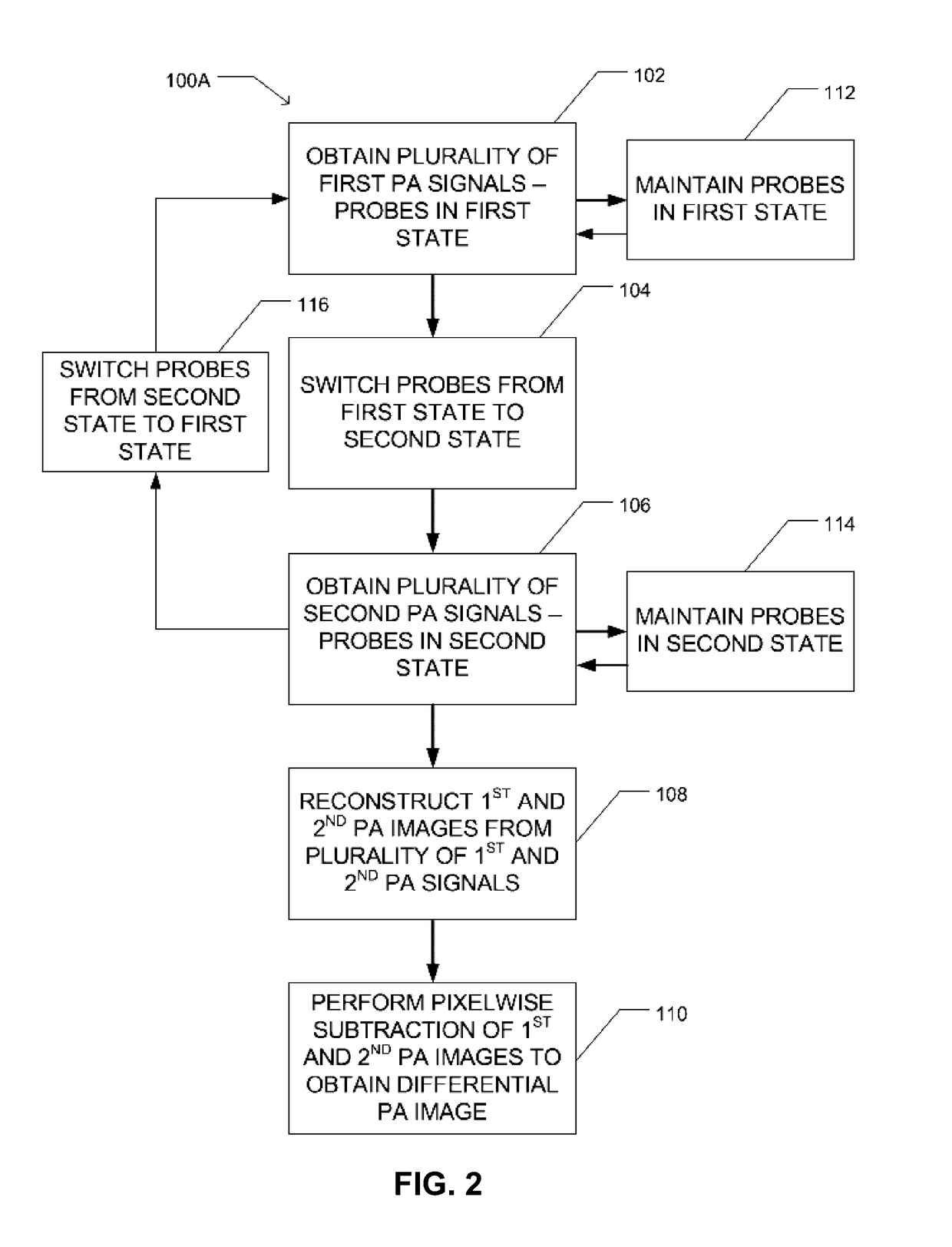
Reversibly switchable photoacoustic imaging systems and methods
a photoacoustic imaging and switchable technology, applied in medical science, health care informatics, surgery, etc., can solve the problems of gfp-like fps lacking strong optical absorption, substantial trade-off, and less well-suited for deep tissue pats
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparison of BphP1 with Available Genetically Encoded Probes
[0173]To assess the characteristics of the bacterial phytochrome BphP1 compared to existing genetically encoded probes (rsTagRFP and iRFP720) for use as reversibly-switchable photoacoustic (RS-PA) probes, the following experiments were conducted.
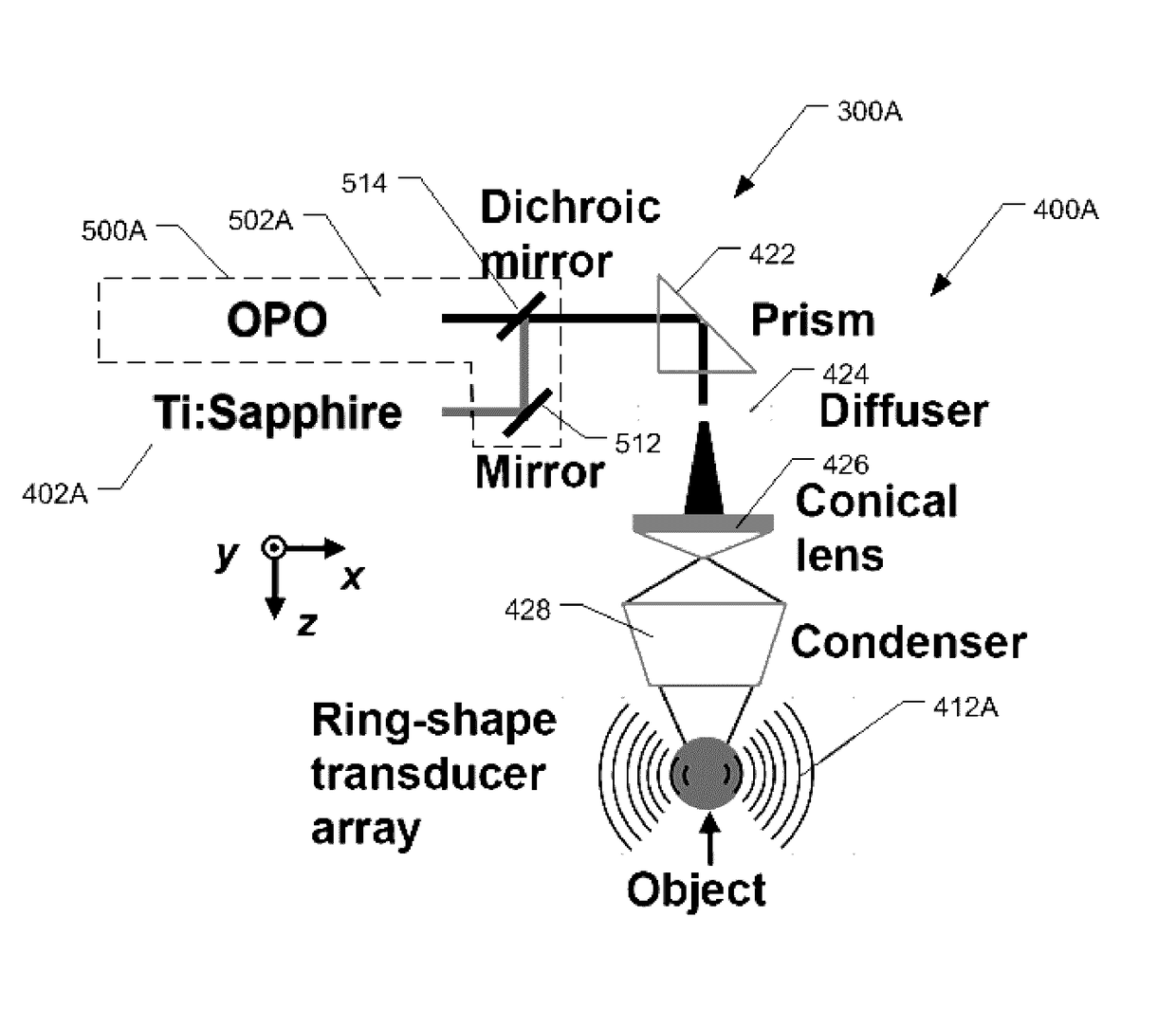
[0174]A whole-body reversibly-switchable PACT system (RS-PACT) was upgraded to utilize BphP1 as a RS-PA probe, as described herein previously and as illustrated in FIG. 5. In this experiment, the RS-PACT system included an optical parametric oscillator (OPO) laser and a Ti:Sapphire laser that were synchronized to provide an excitation optical wavelength range of 400-900 nm. 780 nm light from the Ti:Sapphire laser was used for both whole-body PA imaging and switching off BphP1 at the same time, while the 630 nm light from the OPO laser was used for switching on the protein. The flashlamps of the two pump lasers were synchronized, and the two lasers were individually triggered by an ...
example 2
Effect of Imaging Depth on Reversibly-Switchable Probe
[0179]To assess the ability of BphP1 to function as a RS-PA probe for deep PA imaging in scattering media representative of biological tissues, the following experiments were conducted. Samples of the purified proteins described in Ex. 1 were embedded at depths ranging from 0 mm to 10 mm in a scattering media. The scattering media included 1% intralipid, 10% gelatin, and 7% oxygenated bovine blood in distilled water. The scattering media had a reduced scattering coefficient of about 10 cm−1. The RS-PACT system described in Ex. 1 was used to obtain PA signals at a laser pulse wavelength match to each probe protein's maximum response: 567 nm (rsTagRFP), 715 nm (iRFP720) and 780 nm (BphP1).
[0180]FIG. 18 is a series of PA images of the protein samples obtained as described above at a depths of 0 mm and 10 mm. Each PA image is paired with an image of oxygenated whole bovine blood obtained under matched conditions for comparison. FIG. ...
example 3
Characterization of Reversible Photoswitching of BphP1
[0183]To characterize the reversible photoswitching of BphP1, the following experiments were conducted.
[0184]The optical absorbance of a sample of purified BphP1 at 780 nm was assessed during two transitions: i) a transition from the Pfr (ON) state to the Pr (OFF) state induced by 780 nm illumination; and ii) a transition from the Pr (OFF) state to the Pfr (ON) state induced by 630 nm illumination. FIG. 21 is a graph summarizing the normalized absorbance at 780 nm of the BphP1 over numerous switching cycles. As shown in FIG. 21, the optical absorbance of BphP1 showed an exponential decay from ON to OFF under 780 nm illumination, and an exponential recovery from OFF to ON under 630 nm illumination. For absorbance measurements, a Hitachi U-2000 spectrophotometer was used.
[0185]The BphP1 sample was further assessed during the transition from the Pr (OFF) state to the Pfr (ON) state induced by 630 nm illumination at illumination inte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com