A Process for Producing Hydrogen and Graphitic Carbon from Hydrocarbons
a graphitic carbon and hydrocarbon technology, applied in chemical/physical/physical-chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, physical/chemical process catalysts, etc., can solve the problems of carbon dioxide, not being exploited commercially, harmful to the environment, etc., to achieve higher purity, higher value, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0158]The use of iron ore as the catalyst for the economical production of hydrogen and graphite via the thereto-catalytic decomposition of methane.
Experimental Details
[0159]The present invention provides a method which enables the use of low grade iron oxide as a catalyst for the decomposition of methane. In order to demonstrate the catalytic activity of the low grade iron oxide catalyst of the present invention, samples of low grade iron oxide were compared to high grade iron oxide samples. Two types of high grade iron oxide were tested; hematite (99%, <5 μm, Sigma-Aldrich) and magnetite (95%, <5 μm, Sigma-Aldrich); as well as two iron ore samples: Hematite ore (Pilbara mine) and goethite ore (Yandi mine). The ore samples were milled to <150 μm but otherwise untreated. The ‘as received’ compositional data, particle size distribution, and surface area of all the samples are detailed in Table 1.
TABLE 1Compositional, particle size and surface area data for the iron oxide samples.CaMn...
example 2
[0165]Thermo-catalytic methane decomposition using counter-current MPR.
Counter-Current.
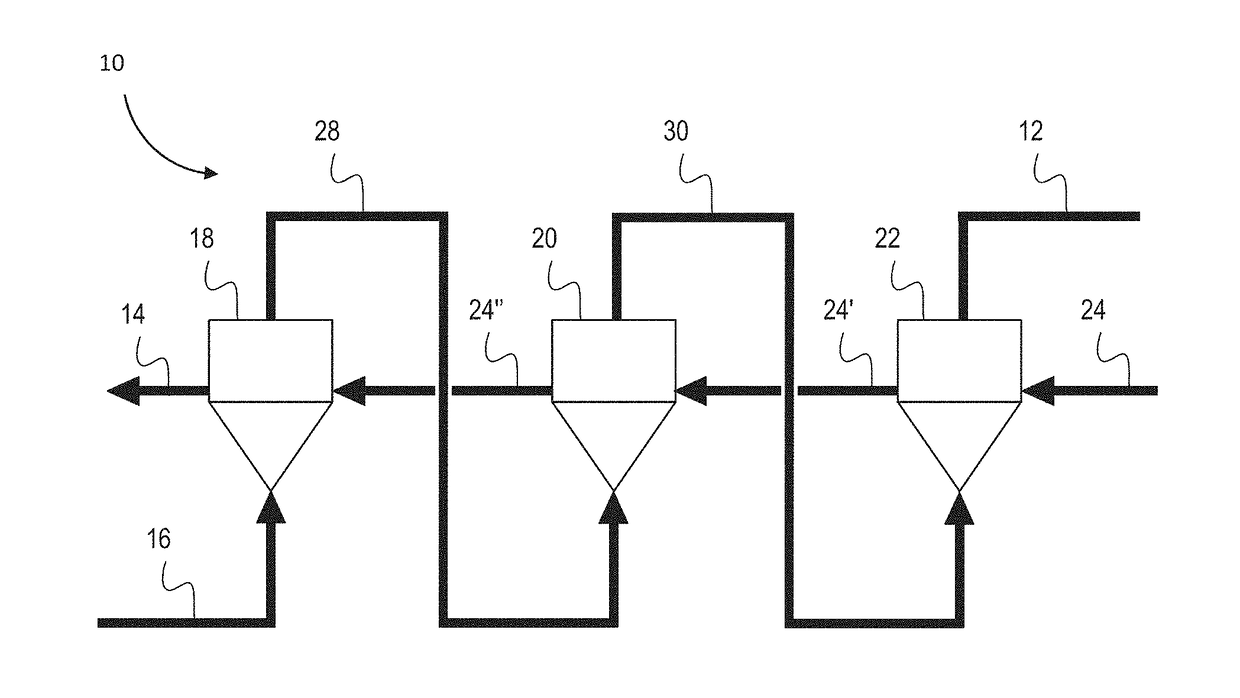
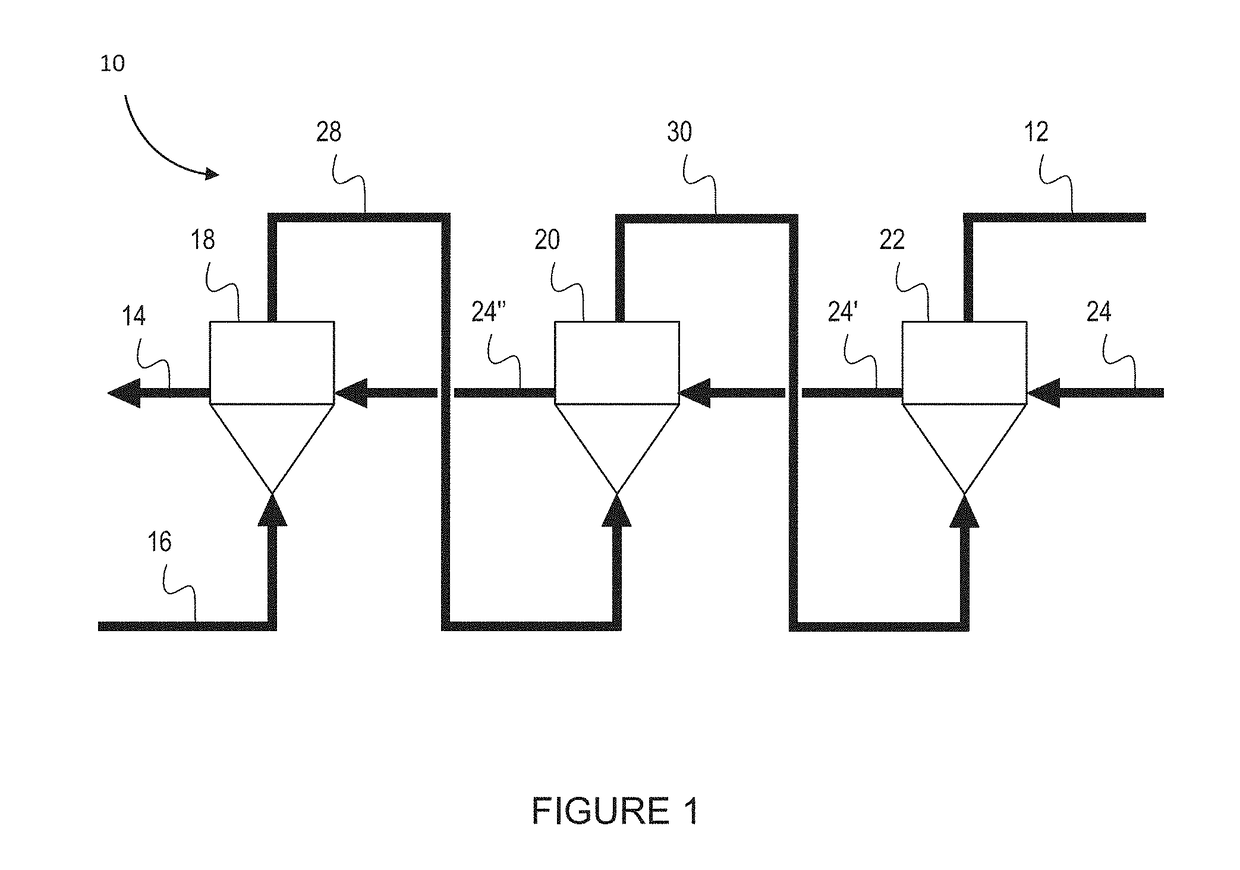
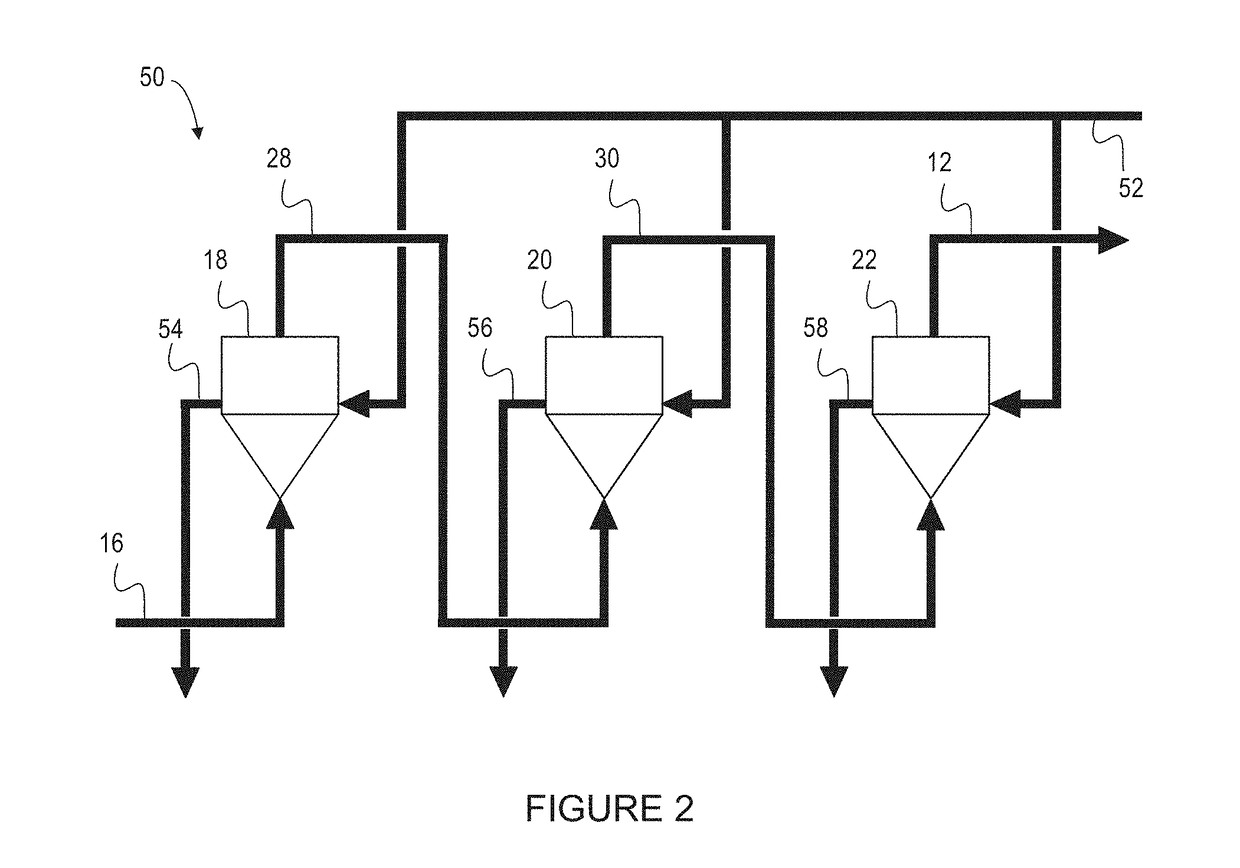
[0166]A three reactor counter-current MPR was set up in a cascade arrangement as shown in the schematic of FIG. 7.
[0167]Experimental evaluation of the counter-current MPR system was undertaken using a static (non-continuous) system. This was done by testing the effect of pressure on the methane conversion efficiency and the carbon yield. The results confirmed that an increase in pressure lowered the methane conversion, and increased the carbon yield, and conversely a lower pressure increase of the methane conversion and lowered the total carbon yield.
Experimental Details
[0168]Effect of reaction pressure on the methane conversion limit.
[0169]The reactor set-up comprised three independent reactor stages (3×½″ OD 316SS Swagelok, 700 mm length) with different set back-pressures (12 bar, 4 bar and atmospheric) and an isothermal temperature of 850° C. Instead of linking the reactors in series, each was ...
example 3
Experimental Details
[0193]Typical low grade iron ore rock consists of distinct sections of high grade iron oxide and low grade counterpart. This type of rock is known as banded iron formation (BIF). A 6.39 g sample of BIF iron ore was prepared, an analysis of the characteristics are shown in Table 3.
TABLE 3Sample AnalysisOXIDESiO2TiO2Al2O3Fe2O3Mn3O4MgOCaONa2OK2OP2O5SO3Iron ore10.620.060.1188.80.010.120.020.230.010.03richsectionIron ore84.10.040.1412.90.070.210.110.030.02poorsectionOXIDECr2O3ZrO2SrOZnOCuONiOBaOPbOL.O.i.TOTALIron ore0.130.020.010.010.271.98102.43richsectionIron ore0.210.43199.26poorsection
[0194]The sample was loaded into a static reactor bed and was contacted at 900° C. with methane gas and atmospheric pressure for a period of 4 hours. Following reaction, the high grade iron oxide band had fragmented whereas the low grade counterpart was largely unaffected.
[0195]Without wishing to be bound by theory it is understood by the inventors that the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com