Modified release pharmaceutical compositions of huperzine and methods of using the same
a technology of huperzine and pharmaceutical compositions, which is applied in the direction of drug compositions, microcapsules, capsule delivery, etc., can solve the problems of higher dose levels of transient dose related nausea and no published data on the efficacy of huperzine, and achieve the effect of better side effect profile and better side effect profil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Dissolution Testing
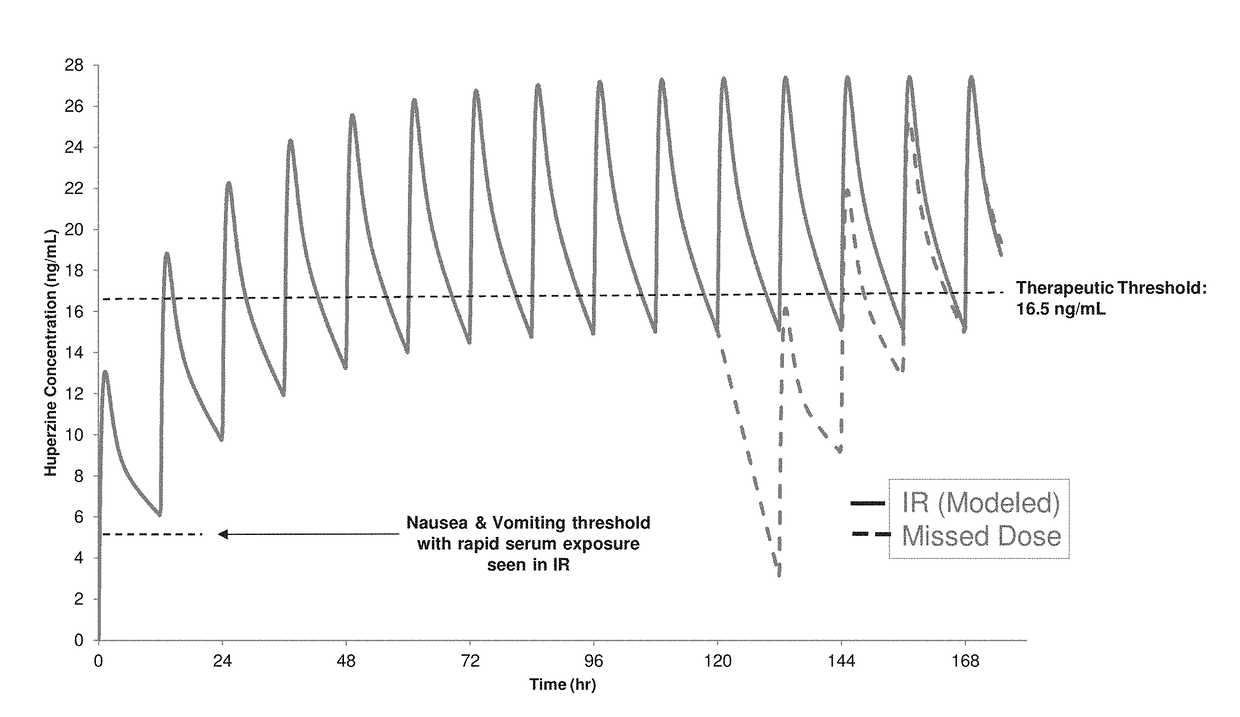
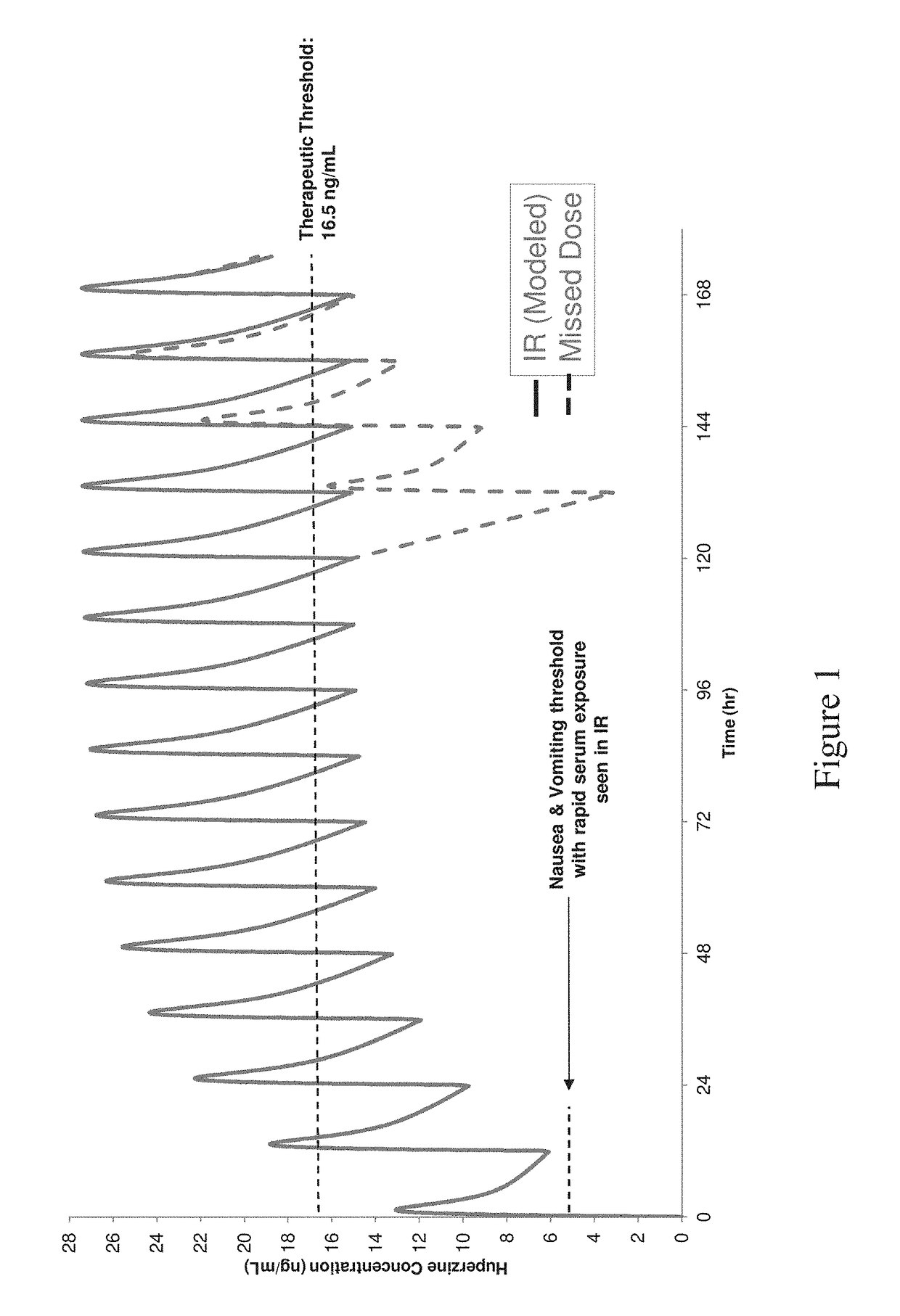
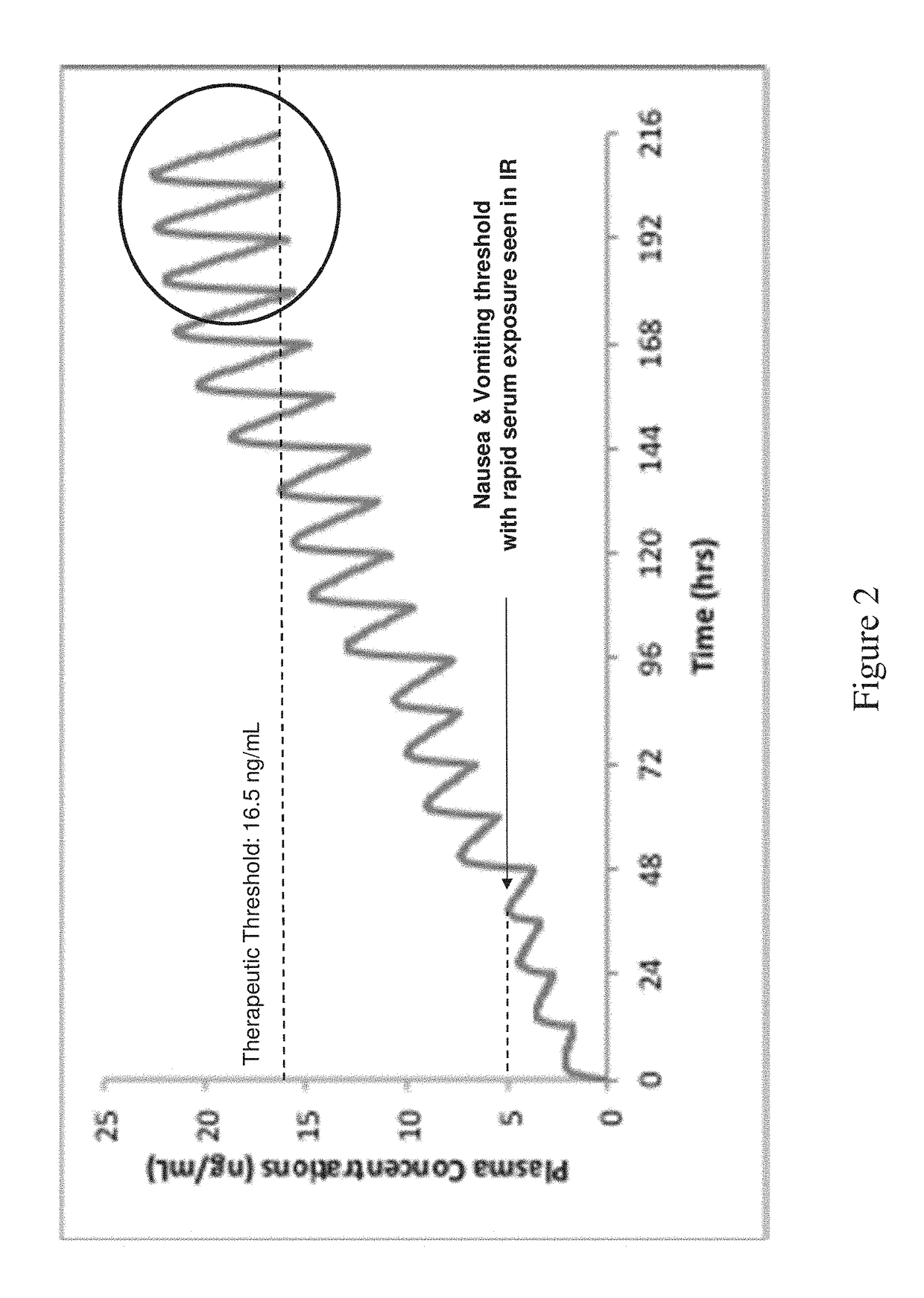
[0188]The compositions were tested according to USP 1 type apparatus at 50 revolutions per minute in 50 mM phosphate (pH 6.8) at 37° C. Results are shown in FIG. 4.
example 2
inetic Study in Dogs
[0189]A Study Objective
[0190]The objective of this study was to determine the plasma pharmacokinetics of modified release huperzine Composition 4A in male beagle dogs. The huperzine A was monitored in plasma for up to 24 hours.
[0191]Vehicle and Pharmaceutical Composition Preparation
[0192]Animals were dosed at a nominal dose of 5.45 mg / kg modified release huperzine A (composition 4A, equivalent to 0.049 mg / kg huperzine A)
[0193]Animal Specifications
[0194]Three, non-naïve, male, beagle dogs (Marshall Bioresources, Beijing, China) weighing ≥6 kg were used in the study. Animals underwent a physical examination for general health by a staff veterinarian prior to assignment to the study. The animals were acclimated to the testing facility prior to the study.
[0195]Environmental Conditions
[0196]Animals were housed in room(s) that were controlled and monitored for relative humidity (targeted mean range 40% to 70%) and temperature (targeted mean range 18° to 26° C.) with 10...
example 3
n of the Bioavailability, Safety and Tolerability of Modified Release Huperzine a Following Multiple Dose Administrations in Healthy Subjects
[0217]A single center, on-site / outpatient, dose escalation study was conducted with oral pharmaceutical composition 4D. The subjects were dosed twice daily (BID) in 4 cohorts of 2 subjects each (Pharmaceutical composition 4D) and 2 cohorts of 3 subjects total (Pharmaceutical composition 4E) to assess plasma levels, safety, and allow necessary dosing alterations to occur prior to dosing any subsequent subjects. The study was conducted in an on-site setting at dose initiation and at times of dose escalation to evaluate safety, and for specimen collection for routine laboratory and pharmacokinetic analysis. Subjects were discharged and compliance of BID dosing was monitored via twice daily phone calls by site staff. The initial dose was 0.5 mg BID with a dose escalation every 2-3 days until a maximum tolerated dose was observed or a maximum of 2.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com