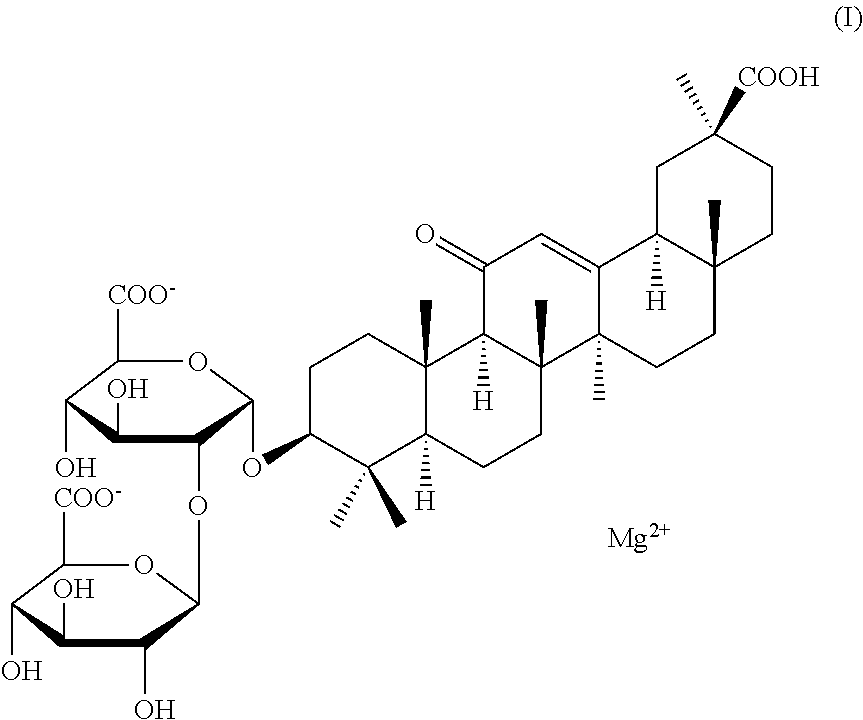
Inhaled Preparation Of Isoglycyrrhizic Acid Or Salt Thereof, And Use In Preparing Drugs For Treating Respiratory System Diseases
a technology of isoglycyrrhizinate and inhalation preparation, which is applied in the field of medicine, can solve the problems of poor patient compliance, poor jaundice fever and liver enlargement accompanying liver damage, and achieves improved bioavailability of magnesium isoglycyrrhizinate inhalation preparation, high fine particle fraction, and high empty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isoglycyrrhizinate Powder for Inhalation
[0074]The magnesium isoglycyrrhizinate was micronized to obtain samples having the following different particle size ranges.
Particle size ofthe active ingredientX10 / μmX50 / μmX90 / μmLarge particle size1.475.7517.24Medium particle size1.385.0812.64Small particle size0.582.035.69
example 1a
[0075]Prescription:
Magnesium isoglycyrrhizinate (large particle size) 1 gLactose A 2 gAmount of preparation100 capsules
[0076]Preparation Process:
[0077]1) The prescribed amount of magnesium isoglycyrrhizinate and the prescribed amount of lactose were taken;
[0078]2) Then sieved and mixed;
[0079]3) The capsules were filled according to 30 mg / capsule, and each capsule contained 10 mg of magnesium isoglycyrrhizinate;
[0080]4) The key quality indicators of powder for inhalation were detected according to requirements of general rule 0111 in the fourth part of the “Chinese Pharmacopoeia”.
example 1b
[0081]Prescription:
Magnesium isoglycyrrhizinate (medium particle size) 1 gLactose A 2 gAmount of preparation100 capsules
[0082]Preparation Process:
[0083]1) The prescribed amount of magnesium isoglycyrrhizinate and the prescribed amount of lactose were taken;
[0084]2) Then sieved and mixed;
[0085]3) The capsules were filled according to 30 mg / capsule, and each capsule contained 10 mg of magnesium isoglycyrrhizinate;
[0086]4) The key quality indicators of powder for inhalation were detected according to requirements of general rule 0111 in the fourth part of the “Chinese Pharmacopoeia”.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com