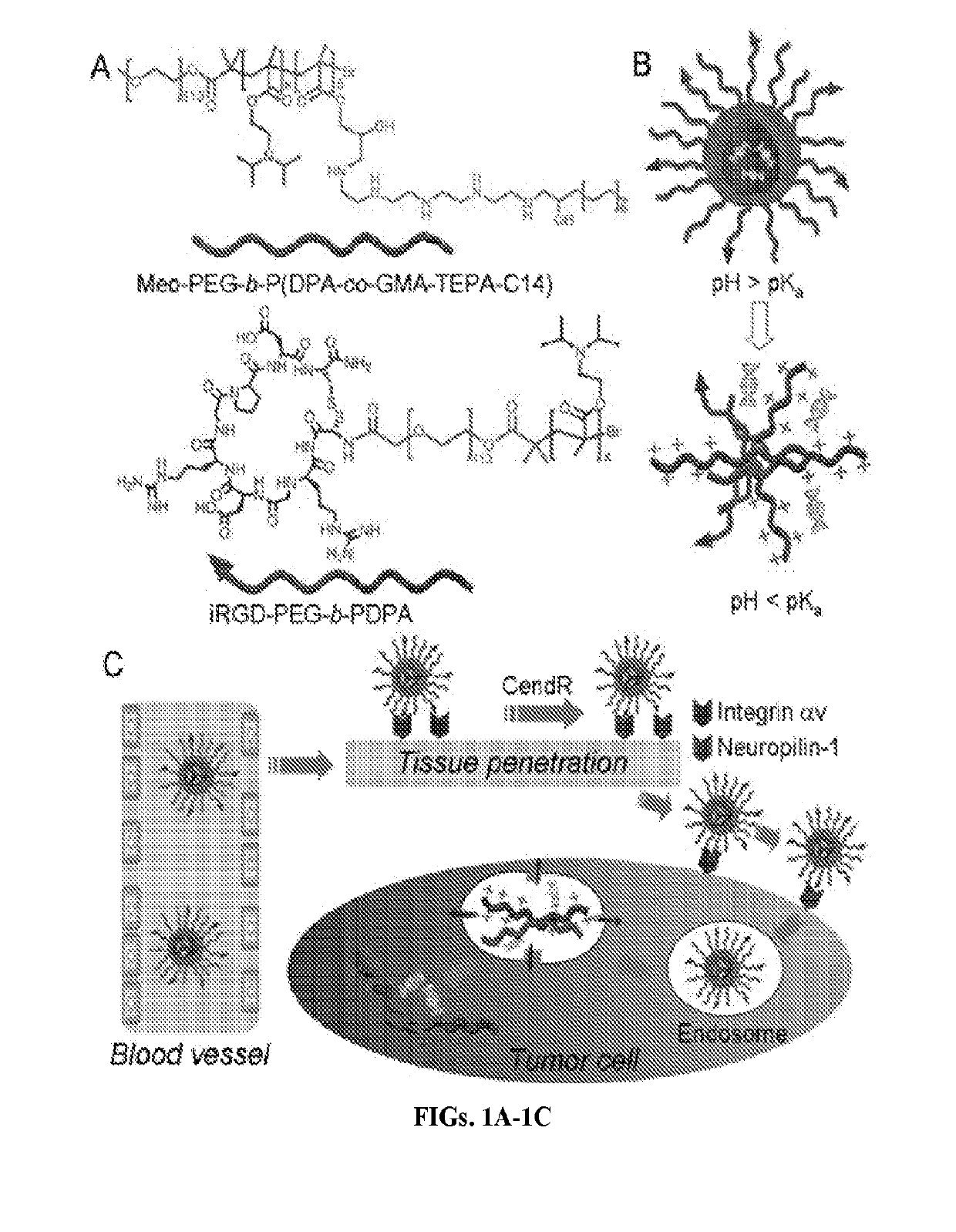
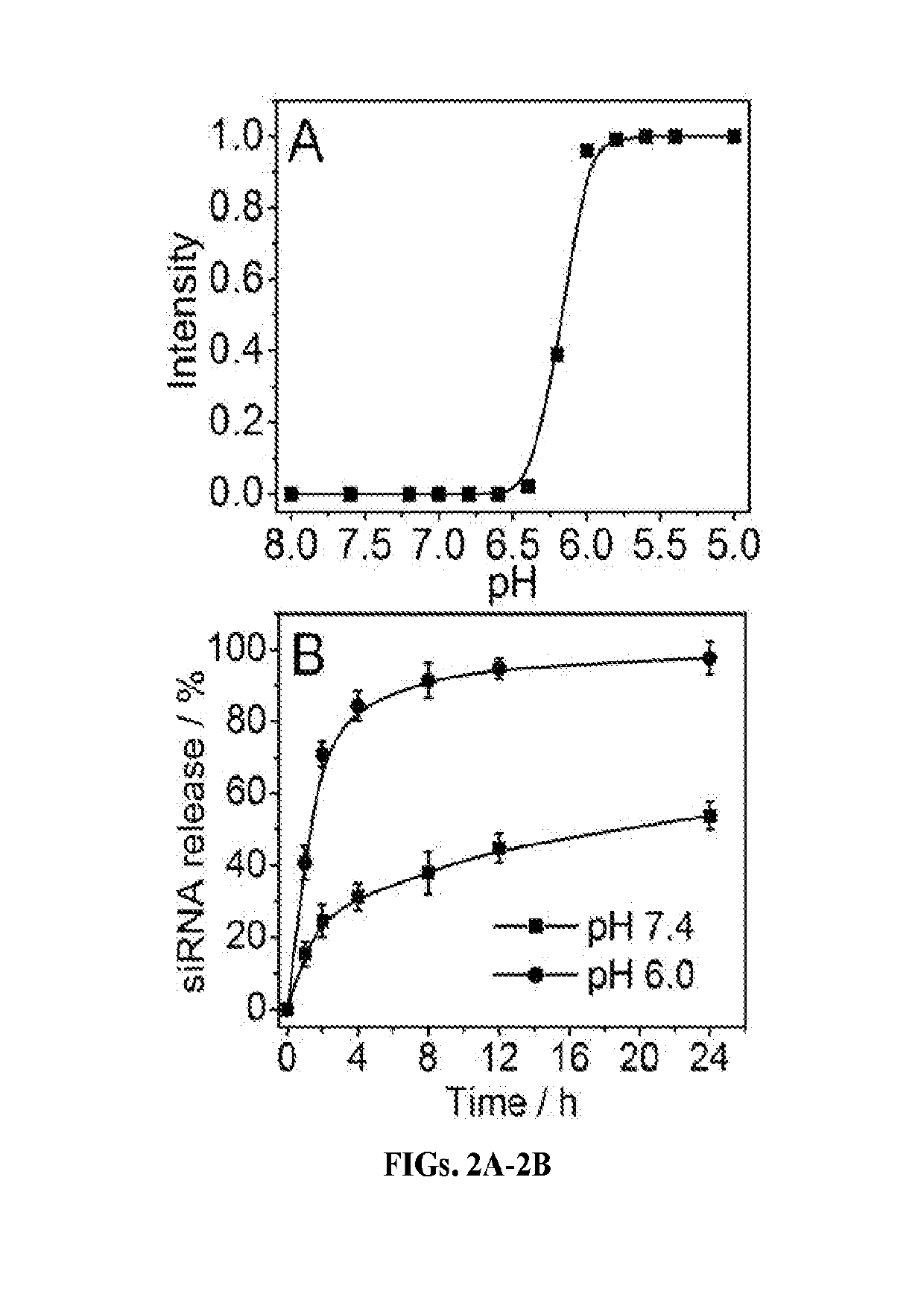
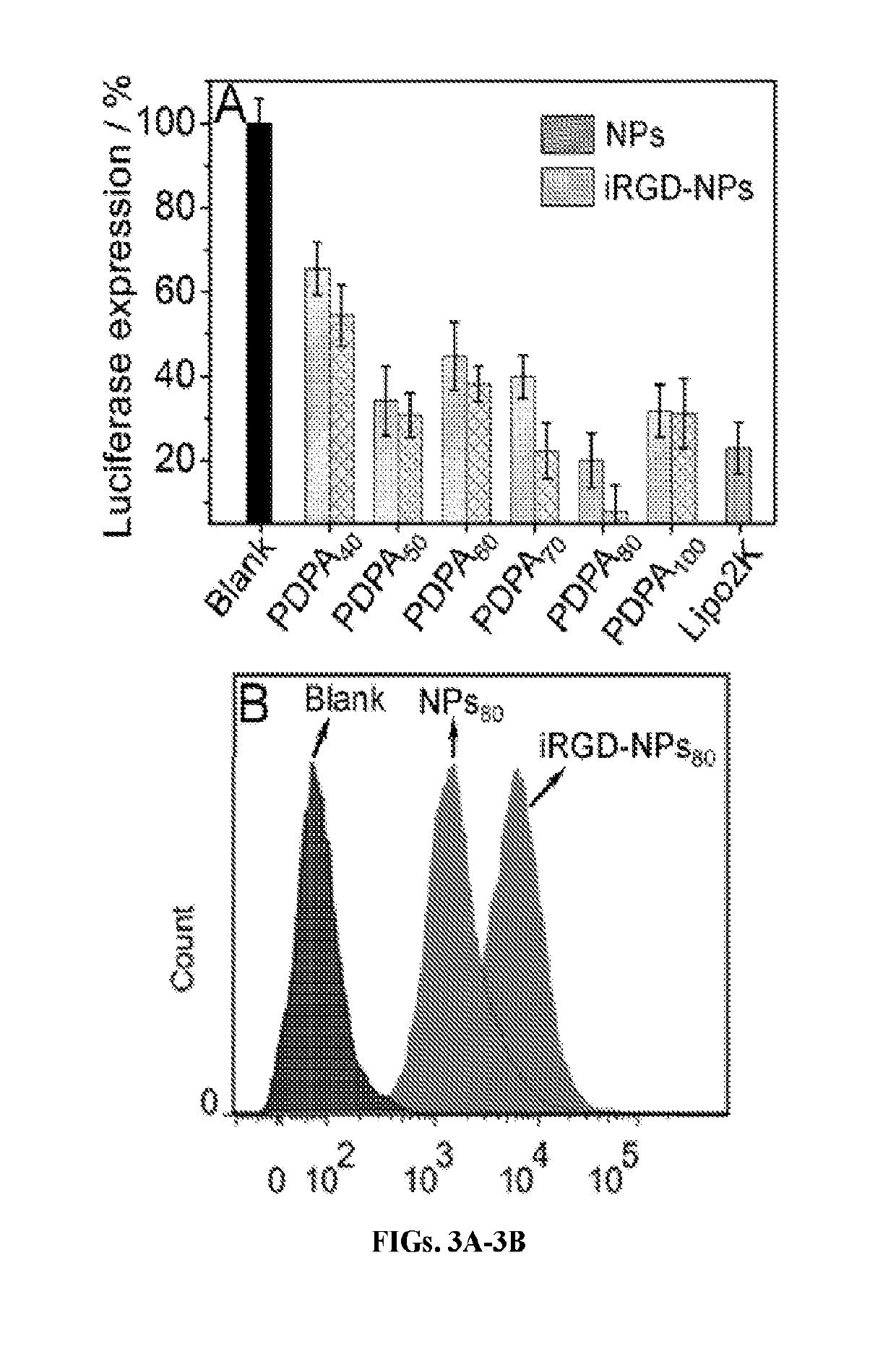
Stimuli-responsive nanoparticles for biomedical applications
a biomedical and stimuli-responsive technology, applied in the direction of nanocapsules, microcapsules, capsule delivery, etc., can solve the problems of affecting the toxic and side effects of healthy tissues, and achieve the effects of high loading efficiency, excellent stability, and high stability in water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Responsive and Tumor-Penetrating Nanoplatform for Targeted siRNA Delivery with Robust Anti-Cancer Efficacy
Methods and Materials
Materials
[0369]Methoxyl-polyethylene glycol (Meo-PEG113-OH) and hydroxyl polyethylene glycol carboxylic acid (HO-PEG113-COOH) were purchased from JenKem Technology and used as received. Internalizing RGD (iRGD) with the sequence CRGDRGPDC (SEQ ID NO:11) was obtained from GL Biochem Ltd. 2-(Diisopropyl amino) ethyl methacrylate (DPA-MA), glycidyl methacrylate (GMA), and methyl methacrylate (MMA) were provided by Sigma-Aldrich and passed over an alumina column before use in order to remove the hydroquinone inhibitors. α-Bromoisobutyryl bromide, triethylamine (TEA), N,N,N′,N′,N′-pentamethyldiethylenetriamine (PMDETA), copper (I) bromide (CuBr), N,N′-dimethylformamide (DMF), tetraethylenepentamine (TEPA), 1,2-epoxyhexadecane, isopropyl alcohol, and dichloromethane (DCM) were acquired from Sigma-Aldrich and used directly. Lipofectamine 2000 (Lipo2K) was purchased...
example 2
Responsive and Tumor-Penetrating Nanoplatform for Targeted siRNA Delivery with Robust Anti-Cancer Efficacy
Methods and Materials
[0442]Materials
[0443]Methoxyl-polyethylene glycol (Meo-PEG113-OH) and hydroxyl polyethylene glycol carboxylic acid (HO-PEG113-COOH) were purchased from JenKem Technology and used as received. Oligoarginine (NH2-Rn—CONH2, n=6, 8, 10, 20, 30) was provided by MIT Biopolymer facility. Allyl protected S,S-2-[3-[5-amino-1-carboxypentyl]-ureido]-pentanedioic acid (ACUPA) was kindly provided by BIND Therapeutics as a gift. 2-(Diisopropyl amino) ethyl methacrylate (DPA-MA) and glycidyl methacrylate (GMA) were provided by Sigma-Aldrich and passed over an alumina column before use in order to remove the hydroquinone inhibitors. α-Bromoisobutyryl bromide, N,N′-dimethylformamide (DMF), triethylamine (TEA), N,N,N′,N′,N′-pentamethyldiethylenetriamine (PMDETA), copper (I) bromide (CuBr), tetraethylenepentamine (TEPA), isopropyl alcohol, p-toluenesulfinate tetrahydrate (PTSF...
example 3
Responsive Nanoparticles (NPs) as Nanoprobe for Cancer Diagnostics
Methods and Materials
Synthesis of Meo-PEG-Br and Br-PEG-COOH
[0507]The detailed synthesis is same as the description in Examples 1 and 2.
Synthesis of methoxyl-polyethylene glycol-b-poly (2-(diisopropylamino) ethylmethacrylate-co-glycidyl methacrylate) (Meo-PEG-b-P(DPA-co-GMA))
[0508]Meo-PEG113-b-P(DPA80-co-GMA5) copolymer was synthesized according to the same method described in Example 1.
Synthesis of Meo-PEG-b-P(DPA-co-GMA-TEPA)
[0509]Meo-PEG-b-P(DPA-co-GMA-TEPA) was synthesized according to the same method described in Example 1.
Synthesis of Meo-PEG-b-P(DPA-co-GMA-TEPA-Cy5.5)
[0510]Meo-PEG-b-P(DPA-co-GMA-TEPA) (0.2 g) and Cy5.5 NHS ester (1.5-fold molar excess relative to the TEPA repeating unit) were well dissolved in 5 mL of THF. After constantly stirring in dark for 48 h, the solution was dialyzed against deionized water and the product was collected after freeze-drying. The synthesis scheme is shown above.
Synthesis ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com