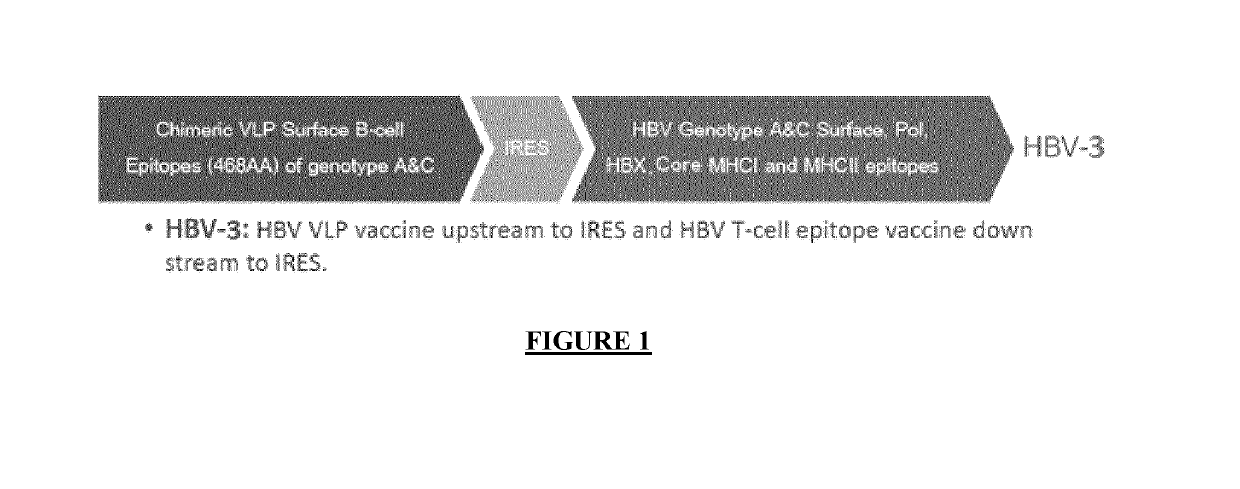
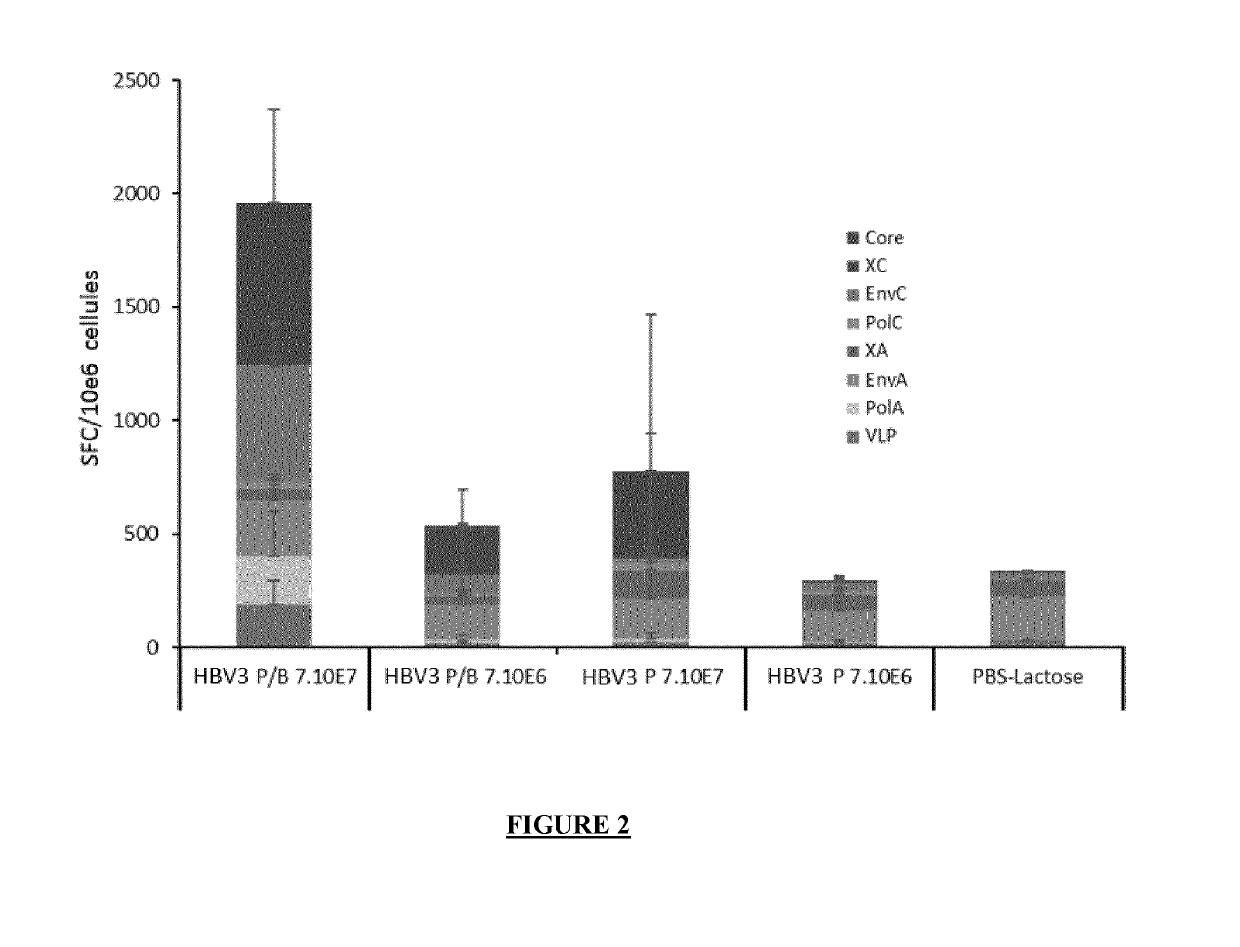
Lentiviral vectors for expression of hepatitis b virus (HBV) antigens
a lentiviral vector and antigen technology, applied in the field of recombinant vaccine technology, can solve the problems of limited likelihood of endogenous promoter activation, insufficient transgene expression and insufficient promoter of mhc class ii promoter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Constructions
[0305]PCR amplification of the proviral region of the pTRIPΔU3-CMV-GFP(15) was performed using direct (5′-CTTACTAGTTGGAAGGGCTAATTCACT CCCAAC-3′; SEQ ID NO: 7) and reverse (5′-CATTCTAGAACTGCTAGAG ATTTTCCACACTG-3′; SEQ ID NO: 8) oligonucleotides encompassing respectively the SpeI and XbaI restriction sites. The resulting fragment was digested and cloned between the SpeI and XbaI sites of the pVAX-1 plasmid (Invitrogen, Lifetech) from which the MluI site have been deleted. The resulting plasmid was named pFLAP-CMV-GFP. The SV40 sequence was amplified by PCR from the pTRIPΔU3-CMV-GFP plasmid (using the 5′-TACCCCGGGCCA TGGCCTCCAAAAAAGCCTCCTCACTACTTC-3′ (SEQ ID NO: 9) and 5′-ACTCCCGGGTAATTTTTTTTATTTATGCAGAGGCCGAG GCCGCC-3′ (SEQ ID NO: 10) oligonucleotides), and cloned into the Pml1 site of the pFLAP-CMV-GFP, the resulting plasmid being then named pFLAP-CMV-GFP-SV. The CMV promoter was amplified with direct (5′-TACACGCGTGGAGTTCCGCGTTACATA ACTTACGG-3′; SEQ ID NO: 11) and revers...
example 2
l Production
[0311]R&D productions: Vectors can be produced by transient calcium-phosphate transfection of HEK 293T as previously describe (25).
[0312]Preclinical and GMP productions: The day before transfection, HEK 293T cells are seeded in culture medium on 24 units of Cell Factory 10 (CF-10, Nunc). Cells are transfected by a calcium-phosphate method as reported previously (25). 18 to 24 hours post-transfection, culture medium is changed with production medium corresponding to Dubelcco's modified Eagle's medium (DMEM / High modified, Hyclone) supplemented with 2% heat-inactivated fetal calf serum (FCS, PAA), 1% L-Glutamine (Gibco by Life technologies), 1% Penicillin-Streptomycin (Gibco by Life technologies), 1% Sodium Pyruvate (Gibco by Life technologies), BENZONASE® (pharma grade I, 100 000 U, Merck Millipore) and MgCL2 1M. Minimum 24 hours after medium renewal, supernatant of the 24 CF-10 is harvested and pooled. After a second BENZONASE® treatment, supernatant is clarified by filtr...
example 3
l Vector Titration
[0314]qPCR reactions: HEK 293T cells are seeded in 6-well plates (BD Falcon) in culture medium and incubated for 4 h at 37° C., 5% CO2 in moist atmosphere. Cells are transduced with 3 successive dilutions of lentiviral vector. 72 h post-incubation, cells are harvested and transduced HEK 293T cell pellets are produced. Total genomic DNA from transduced cell-pellets is extracted using a method based on QIAGEN QIAamp DNA mini kit handbook. Proviral quantification is performed using Taqman qPCR. The amplification is performed with the Master Mix (Fermentas Thermo Scientific), the Flap A (CCCAAGAACCCAAGGAACA; SEQ ID NO: 18) and Flap S (AGACAA GATAGAGGAAGAGCAAAAC; SEQ ID NO: 19) primers and LENTI TM probe (6FAM-AACCATTAGGAGTAGCACCCACCAAGG-BBQ; SEQ ID NO: 20). Normalization is performed with the quantification of the actin gene (same Mix, Actine A-CGGTGAGGATCTTCATGAGGTAGT-(SEQ ID NO: 21), Actine S-AACACCCCAGCCATGTACGT-(SEQ ID NO: 22) primers and HUMURA ACT TM probe-6FAM-C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com