Olive derived cell culture and methods for preparing and using the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Olive Cell Lines (Calli) and Suspension Cultures in Erlenmeyer
1. Material and Methods
Plant Material
[0151]Olive cell culture was initiated from olive fruits (Olea europaea L.) of Nabali, Manzanilla, Souri and Barnea cultivars, including all leaves, petioles, fruits and kernels.
Establishment of Calli from Olive Explants
[0152]Olive plant parts: young leaves and their petriols, immature fruits (about 6 weeks from flowering) and kernels from immature olives were rinsed under running water and sterilized by agitation in 70% ethanol for three minutes and then 3% Na-hypochlorite solution for 20 minutes, followed by three washes in sterile water. Plant parts were dried under sterile conditions and further dissected into ˜0.5 cm sections.
[0153]Olive plant explants were plated on MS Murashige and Skoog medium basal medium (Table 1B) supplemented with sucrose and various combinations of the auxines 2,4-dichlorophenoxyacetic acid (2,4-D), 1-naphthaleneacetic acid (NAA) and the cyto...
example 2
Expression Polyphenolic Compounds in Olive Cell Calli
Materials and Methods
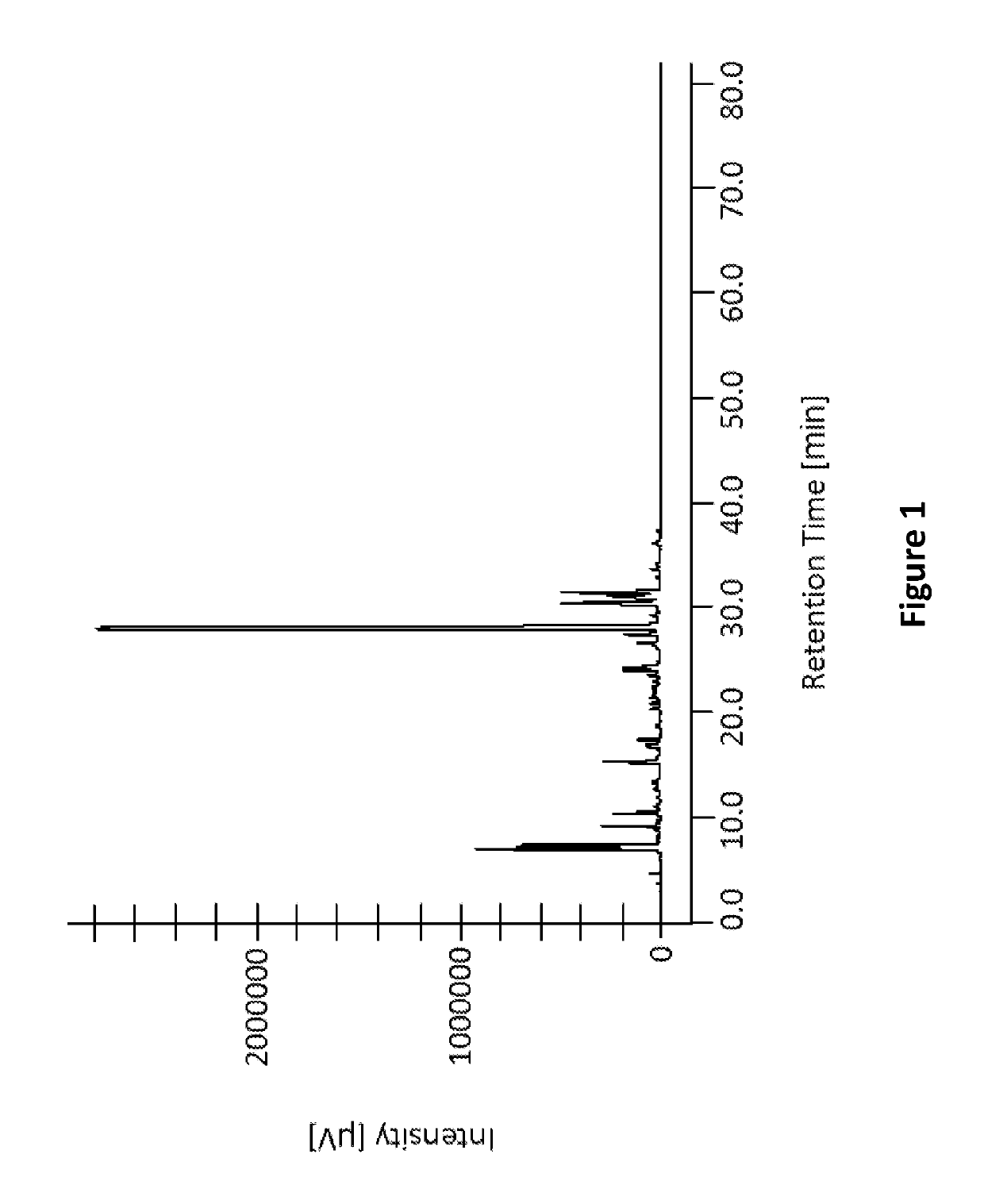
[0160]Polyphenols extraction for HPLC analysis: Fresh olive Callus cell cultures were extracted for analytical determination of polyphenol content in the olive culture. About 1 gr of callus was harvested and kept at −20° C. for at least 16h before analysis. The callus was extracted by 80% methanol / water solution in a ratio of 0.4 ml methanol per 1 gr of cells. The suspension was sonicated for 10 minutes at 30° C. in a sonicator. The solution was centrifuged and the supernatant was re-centrifuged, filtered through a 0.45 μm filter and used for the HPLC analysis.
[0161]Olive tissues (fruits and leaves) were crushed under liquid nitrogen and extracted as described above, the extract was analyzed by HPLC and used as a reference for olive polyphenols content.
[0162]Olive calli extract was analyzed by LC-MS. The method is described in: Food Chemistry 138 (2013) 1381-1391.
[0163]Olive calli samples were extracted in met...
example 3
[0169]Scale Up of Olive Culture in Bioreactors and Testing of the Total Amount of the Polyphenols, including, Hydroxytyrosol, Tyrosol, Oleuropein, Verbascoside and Pinoresinol Content in Olive Cells Grown in Large Scale Bioreactors
Materials and Methods
Stage 1: Cells are Prepared and Grown as Described in Example 1.
Stage 2: Small Scale Bioreactor
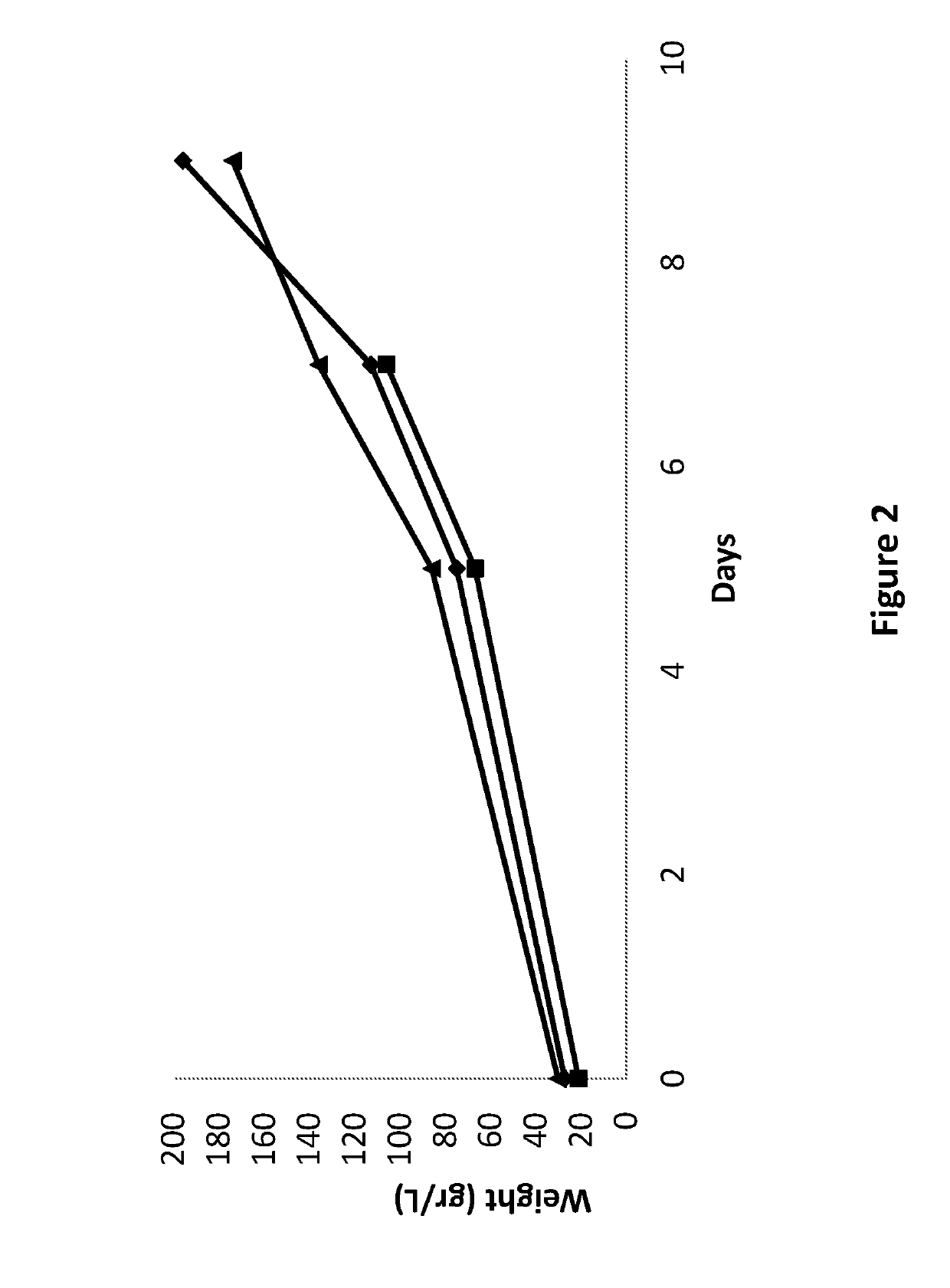
[0170]Small scale bioreactor culturing is performed by inoculating a 7 to 16 old day cell suspensions grown in the Erlenmeyer of Stage 1 into a 4-8 liter disposable bioreactor at 25+5° C. The cells were grown in the suspension under continuous fluorescent light (1000 1×) in a growing medium containing modified MS supplemented with sucrose 1-6% and the Kinetin, 2,4,D, NAA, BA, 2iP and casein hydrolysate or a combination thereof
[0171](pH 4.0-6.0)-. The cells were sub-cultured every 5-21 days.
Stage 3: Large Scale Bioreactor
[0172]The cell suspensions grown in a small scale bioreactor were inoculated into a 30-50 liter disposable bioreactor. The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com