Ion exchange membrane, method for producing ion exchange membrane and electrolyzer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
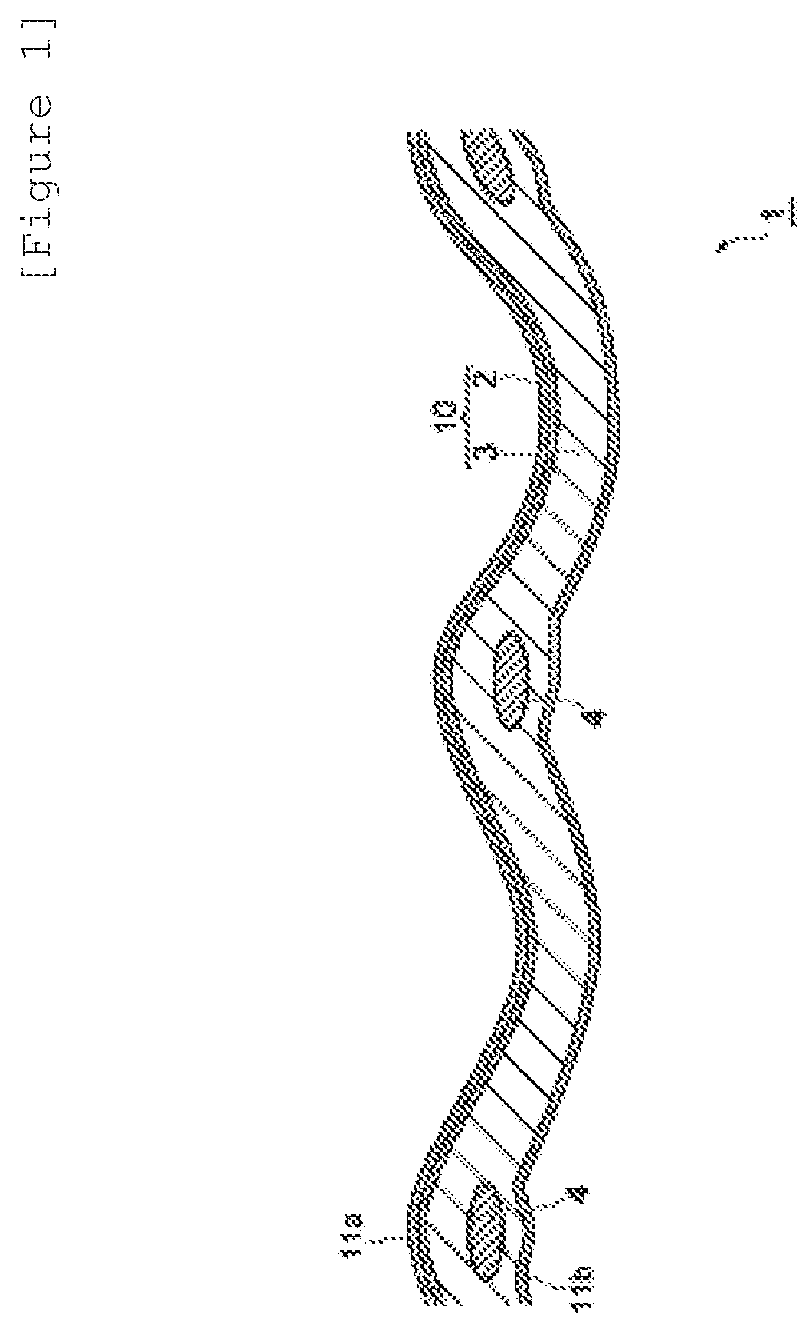
[0140]As reinforcement yarn, a yarn-like material prepared by twisting 100-denier tape yarn made of polytetrafluoroethylene (PTFE) at 900 turns / m (hereinafter, referred to as PTFE yarn) was used. As warp sacrifice yarn, yarn prepared by twisting polyethylene terephthalate (PET) of 35 deniers and 8 filaments at 200 turns / m (hereinafter, referred to as PET yarn) was used. As weft sacrifice yarn, yarn prepared by twisting polyethylene terephthalate (PET) of 35 deniers and 8 filaments at 200 turns / m (hereinafter, referred to as PET yarn) was used. First, plain-weaving was carried out with the PTFE yarn arranged at 24 strands / inch and two strands of the sacrifice yarn arranged between adjacent strands of the PTFE yarns to thereby obtain woven fabric having a thickness of 100 μm.
[0141]Then, provided were a polymer (A1) as a dried resin, which was a copolymer of CF2═CF2 and CF2═CFOCF2CF(CF3)OCF2CF2COOCH3 and had an ion exchange capacity of 0.85 mg equivalent / g and a polymer (B1) as a dried...
example 2
[0148]An ion exchange membrane was prepared in the same manner as in Example 1 except that the amount of HS-210 used was reduced and the viscosity was changed to 12.0 mPa-s in Example 1. In this ion exchange membrane, the content of the fluorine-containing polymer in the binder was 100% by mass.
[0149]The distribution density was measured in the same manner as in Example 1, and the result was 0.5 mg per 1 cm2. The coverage was measured in the same manner as in Example 1, and the result was 56.5%.
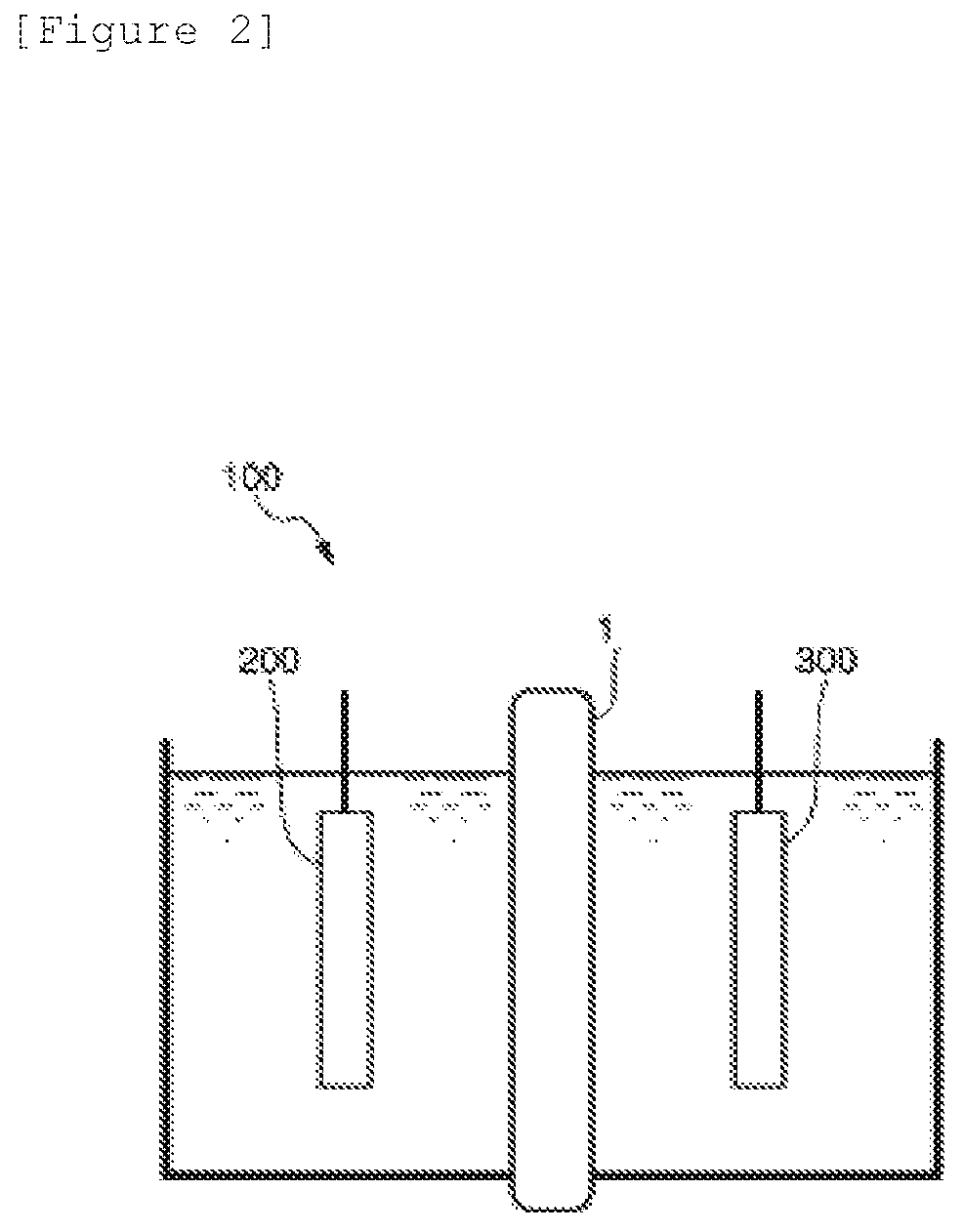
[Electrolysis Evaluation]
[0150]As a result of electrolysis performance evaluation on this ion exchange membrane under the same conditions as in Example 1, the voltage indicated was as low as 3.08 V. As a result of test on resistance to impurities, the decrease in the current efficiency was as low as 0.741 / day, and high durability to impurities was indicated.
example 3
[0151]An ion exchange membrane was prepared in the same manner as in Example 1 except that the amount of HS-210 used was raised and the viscosity was changed to 8.5 mPa-s in Example 1. In this ion exchange membrane, the content of the fluorine-containing polymer in the binder was 100 h by mass.
[0152]The distribution density was measured in the same manner as in Example 1, and the result was 0.5 mg per 1 cm2. The coverage was measured in the same manner as in Example 1, and the result was 92.9.
[Electrolysis Evaluation]
[0153]As a result of electrolysis performance evaluation on this ion exchange membrane under the same conditions as in Example 1, the voltage indicated was as low as 3.06 V. As a result of test on resistance to impurities, the decrease in the current efficiency was as low as 0.42% / day, and high durability to impurities was indicated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com