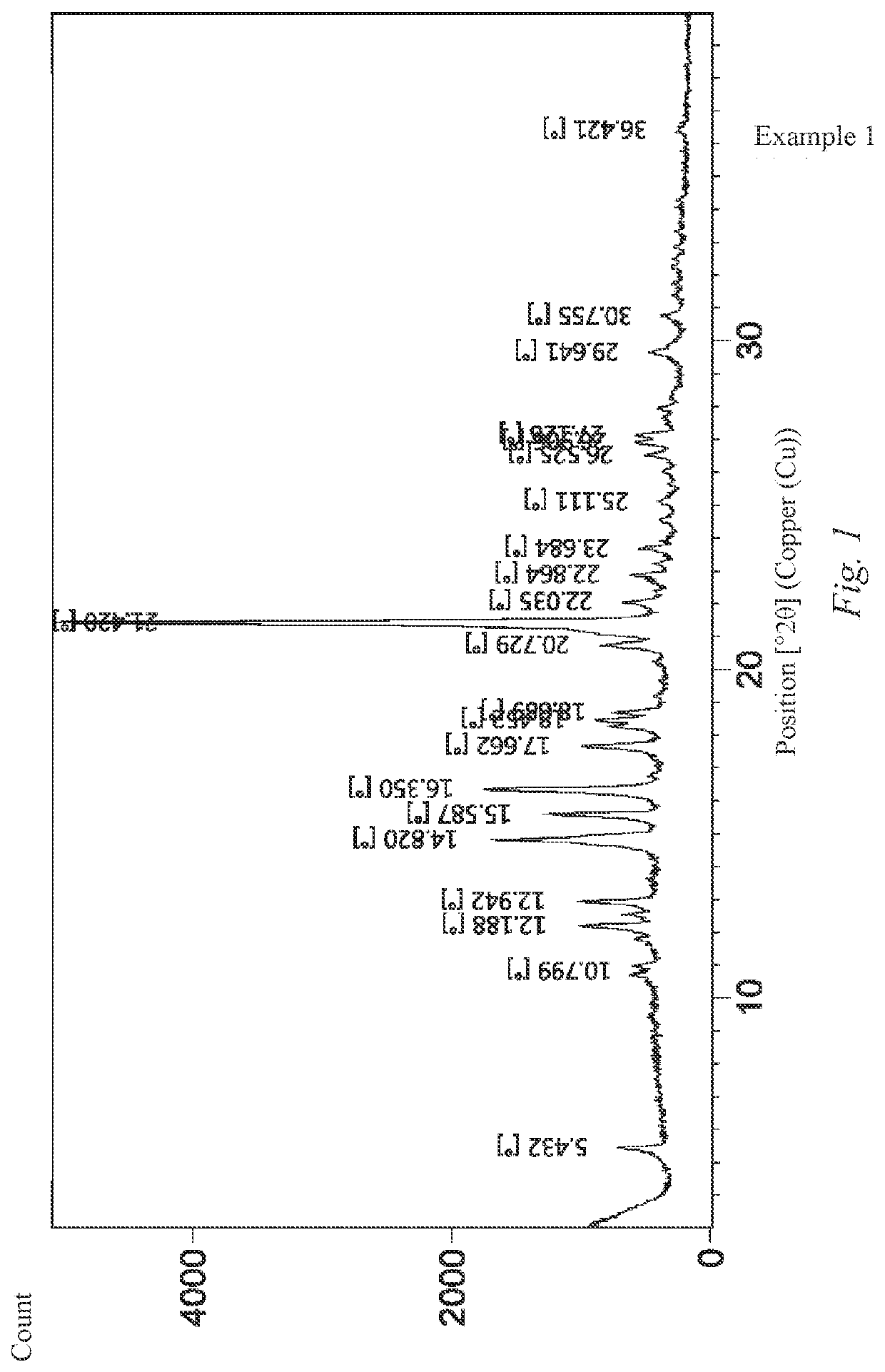
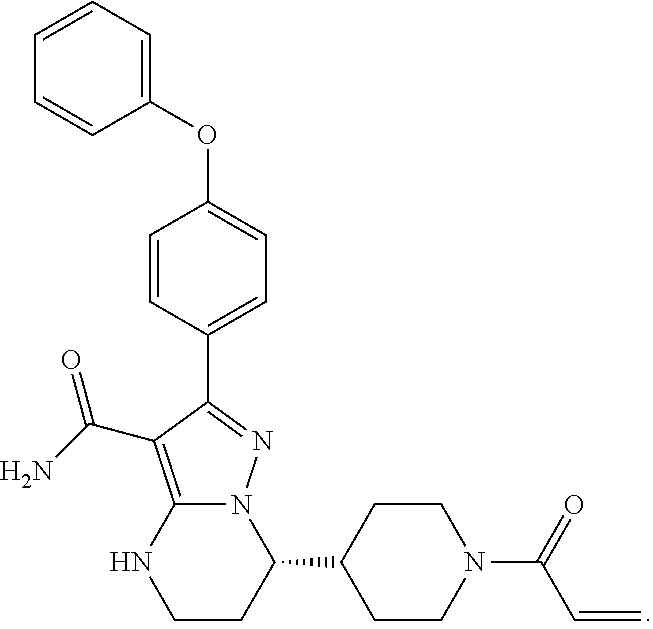
Oral solid tablet comprising bruton's tyrosine kinase inhibitor and preparation method therefor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0048]Preparation of Solid Tablet for Oral Administrations of Zanubrutinib, Specification: 160 mg
[0049]Prescription (Per 100 g Plain Tablets):
Zanubrutinib (Crystal form A)34.8gLactose53.2gCroscarmellose sodium2gColloidal silica4.5gSodium lauryl sulfate1gMicrocrystalline cellulose4gMagnesium stearate0.5gOpadry2.4g
[0050]Preparation Process: 53.2 g of lactose, 2 g of croscarmellose sodium, 1 g of sodium lauryl sulfate and 34.8 g of Zanubrutinib are added into a high-shear granulator (MYCROMIX, manufactured by BOSCH) and mixed for 5 minutes, an appropriate amount of purified water is added for granulation, followed by dying and then sizing, 4.5 g of colloidal silica, 4 g of microcrystalline cellulose and 0.5 g of magnesium stearate are further added and mixed. After mixing, the above ingredients are pressed into tablets to obtain plain tablets. The above-mentioned plain tablets are coated with 2.4 g of Opadry to obtain solid tablet for oral administrations containing Zanubrutinib.
[0051]...
example 2
[0053]Preparation of Solid Tablet for Oral Administrations of Zanubrutinib, Specification: 160 mg
[0054]Prescription (Per 100 g Plain Tablets):
Zanubrutinib (Crystal form A)36.2gLactose55.6gCroscarmellose sodium2.2gColloidal silica4.3gSodium lauryl sulfate1.1gMagnesium stearate0.5g
[0055]Preparation Process: 55.6 g of lactose, 2.2 g of croscarmellose sodium, 1.1 g of sodium lauryl sulfate, 4.3 g of colloidal silica and 36.2 g of Zanubrutinib are added into a high-shear granulator and mixed for 5 minutes, an appropriate amount of purified water is added for granulation, followed by dying and then sizing, and 0.5 g of magnesium stearate is further added and mixed. After mixing, the above ingredients are pressed into tablets to obtain plain tablets, that is, an solid tablet for oral administration containing Zanubrutinib.
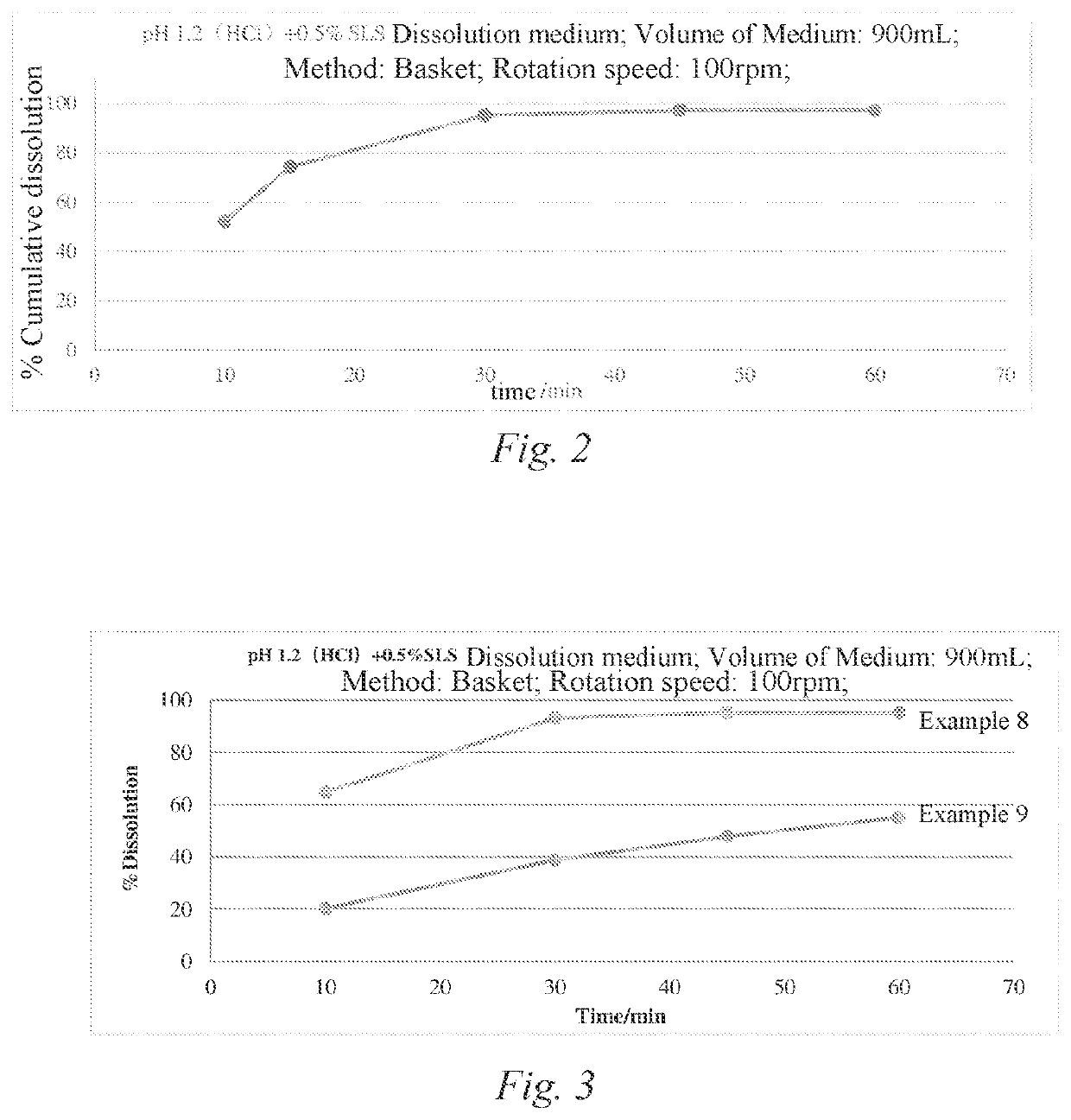
[0056]Drug Cumulative Dissolution (In Vitro Dissolution) Test: About 90% of Zanubrutinib is dissolved at 30 minutes.
example 3
[0057]Preparation of Solid Tablet for Oral Administrations of Zanubrutinib, Specification: 160 mg
[0058]Prescription (Per 100 g Plain Tablets):
Zanubrutinib (Crystal form A)33.3gLactose49.2gHypromellose2gCroscarmellose sodium2gColloidal silica4gSodium lauryl sulfate1gMicrocrystalline cellulose8gMagnesium stearate0.5g
[0059]Preparation Process: 49.2 g of lactose, 2 g of croscarmellose sodium, 1 g of sodium lauryl sulfate and 33.3 g of Zanubrutinib are added into a high-shear granulator and mixed for 5 minutes, 2 g of hypromellose aqueous solution is added for granulation, followed by dying and then sizing, 4 g of colloidal silica, 8 g of microcrystalline cellulose and 0.5 g of magnesium stearate are further added and mixed. After mixing, the above ingredients are pressed into tablets to obtain plain tablets, that is, an solid tablet for oral administration containing Zanubrutinib.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com