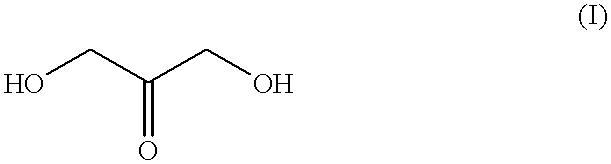
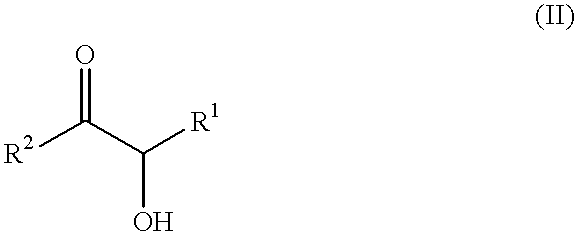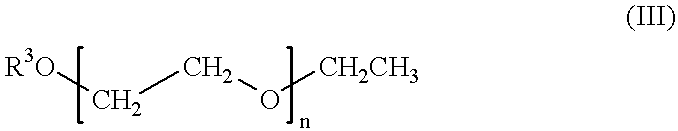Self-tanning dihydroxyacetone formulations having improved stability and providing enhanced delivery
a technology of dihydroxyacetone and formulation, applied in the direction of body powder, hair cosmetics, pharmaceutical delivery mechanisms, etc., can solve the problems of ph of the formulation mixture soon beginning to drop, mixture becoming more acidic, and reversed chemical instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Self-Tanning Cosmetic Formulation in Spray Form for Producing a Dark Tan
The individual components of the spray formulation, identified in Table 1 below, were brought together in four phases, i.e., four different groups of components were added separately to the vessel in which the formulation was being prepared largely as a matter of convenience. As a practical matter, all of the components of the formulation were brought into admixture with each other in a single step, and the order of addition was not critical, but was, rather, also a matter of convenience.
example 2
Self-Tanning Cosmetic Formulation in Spray Form for Producing a Deep Dark Tan
The individual components of the spray formulation, identified in Table 2 below, were brought together in essentially the same manner as above-described in Example 1. Four phases of components were added separately to the vessel largely as a matter of convenience. The darker tan produced by the formulation of this example, compared to that produced by the formulation of Example 1 above, is the result of 50% more of the active self-tanning agent, dihydroxyacetone, present in this example.
example 3
Self-Tanning Cosmetic Formulation in Oil-in-Water Emulsion Cream Form for Producing a Deep Dark Tan
The continuous water phase was first heated to a temperature of 75.degree. to 80.degree. C., after which the components of Phase I set out in Table 3 below, were admixed with the water and dispersed therein under high-speed agitation. Next, the temperature of the water was restored to 75.degree. C., after which the Phase II components set out in Table 3 were added to the composition mixture under moderate agitation, and mixing continued for 10 to 30 minutes. Thereafter, the composition mixture was actively cooled to 35.degree. to 45.degree. C., after which the components of the remaining Phases III, IV and V set out in Table 3 were combined and added to the composition mixture. The composition mixture was then actively cooled to 30.degree. C., after which the fragrance phase was added to the composition mixture, to which water was then added in sufficient quantity to reach the concentr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelengths | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com