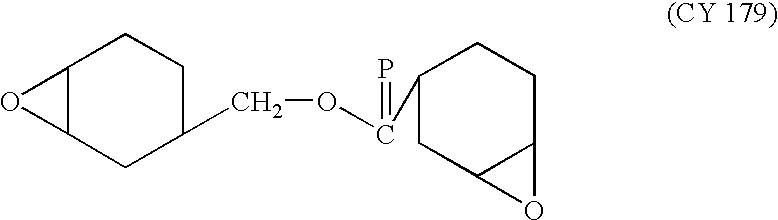
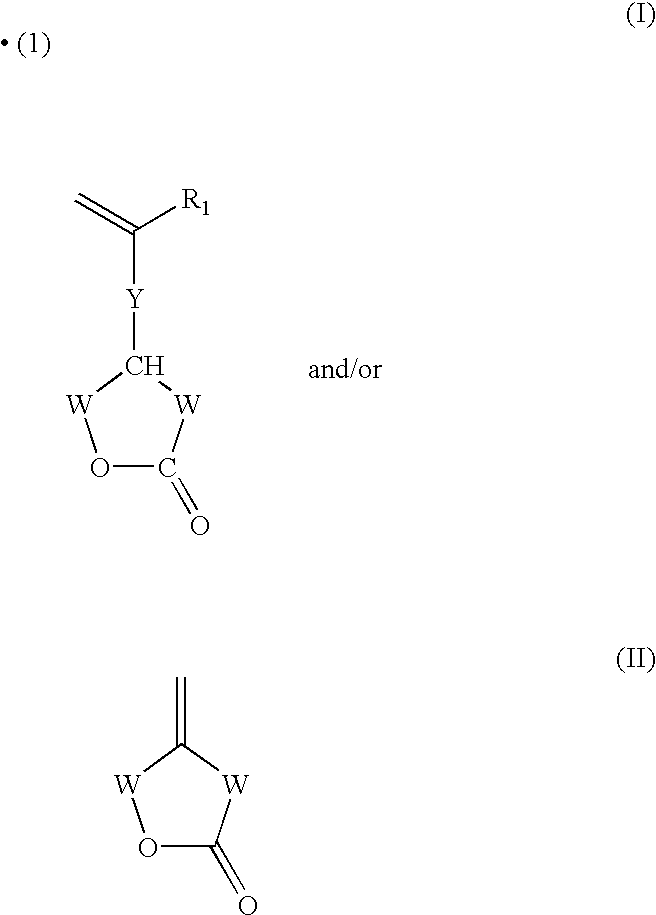
Composition (e. g. ink or varnish) which can undergo cationic and/or radical polymerization and/or crosslinking by irradiation, based on an organic matrix, a silicone diluent and a photoinitiator
a technology of photopolymerization and organic matrix, applied in the direction of metal/metal-oxide/metal-hydroxide catalyst, physical/chemical process catalyst, instrument, etc., can solve the problems of turbidity, coloured and thus non-transparent appearance, and disrupt the polymerization mechanism,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The diluent a) is fully miscible in the epoxy matrix (M1) or the polyol matrix (T1).
The behaviour of the crosslinking under ultraviolet light of different formulations in the presence of a sulphonium borate of formula (P1) is studied.
The three formulations prepared comprise:
(F1) 5parts(a)95parts(M1)(F2)10parts(a)90parts(M1)(F3)20parts(a)80parts(M1)(CF) control formulation100parts(M1)
0.5 p of a wetting agent based on polyether silicone, sold by the company OSI (Silwet L7640 or L7644) and a solution of photoinitiator in vinyl lactate are added to these formulations so as to have a given concentration, the value of which is specified later in Table I.
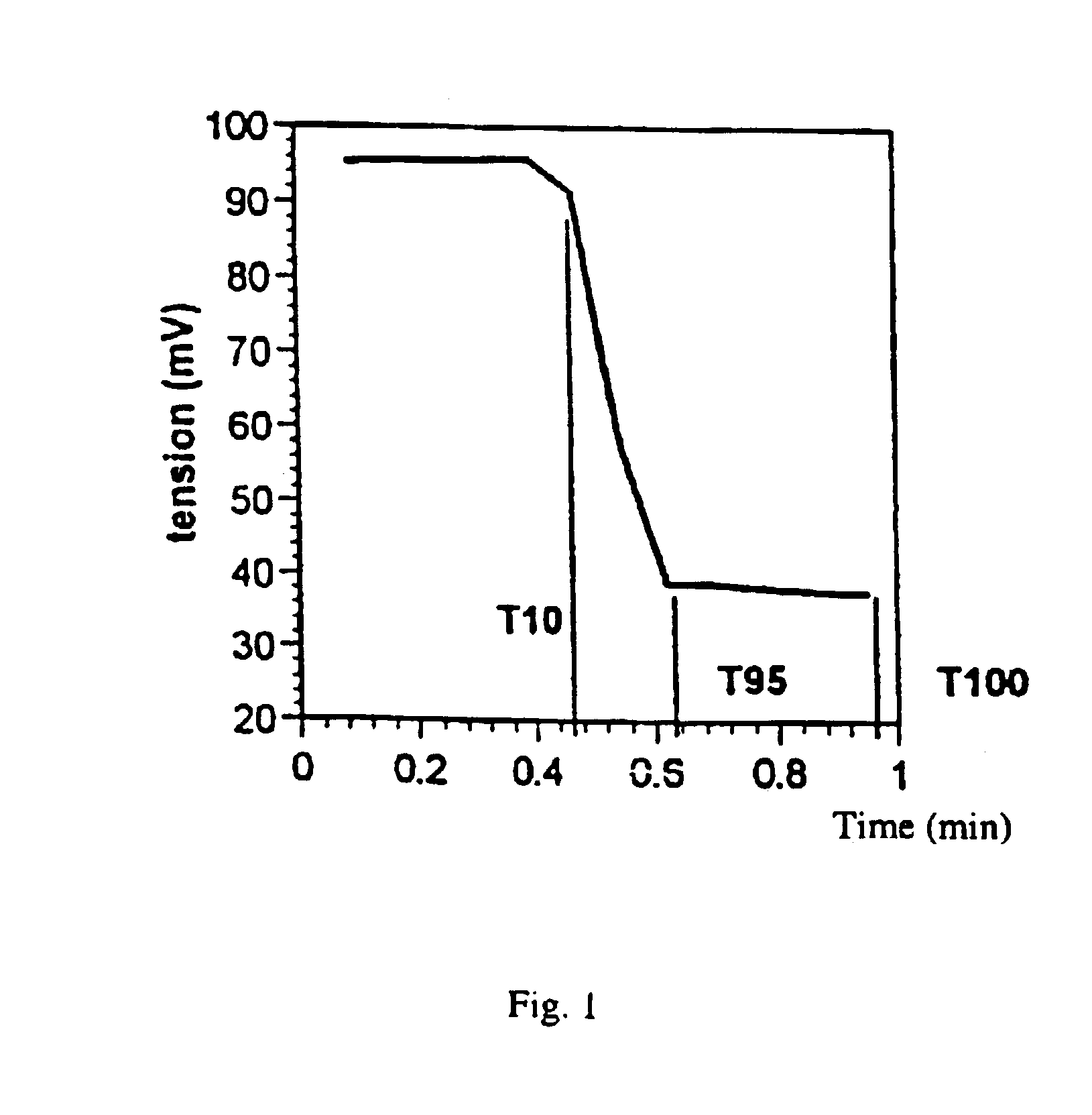
The crosslinking is studied in a thick layer (RAPRA (V. N. C.)) and as a thin layer. 5, 10 and 20 μm films are prepared using threaded coating bars (Bar 0, 2 or 3) on an aluminium support. A few drops of composition are used and the aluminium support is degreased by cleaning with propanol or isopropanol.
Crosslinking tests with a vibrating ...
example 2
The rate of crosslinking and the nature of the network obtained after adding T1 to the matrix (A) in addition to M1, which makes it possible to improve the flexibility of the resin after crosslinking, is now compared.
The ratio [OH] / [epoxy]=¼.
The new control formulation (C′F) becomes:
85parts of(M1),15parts of(T1).Various amounts of silicone diluent (a) are added:(F′1)80.75parts of(M1),14.25parts of(T1),5parts of(a),(F′2)76.5parts of(M1),13.5parts of(T1),10parts of(a),(F′3)68parts of(M1),12parts of(T1),20parts of(a).
0.5 p of the same wetting agent (Silwet L7640) as in the preceding example and a fixed known amount of photoinitiator are added.
The same lamps as previously are used.
The results obtained during the various tests are collated in Table II below.
TABLE IILampImpactPencil“H”strengthhardness / TestPhoto-(Fusion)MEK24 hPersozFilm (e μm)initiator(120 W / cm)*(test)**inch-2124 hF′1 (20 μm)5 × 10−3 3 m / min:1 h / 10015H / 269mol / l1 pass.F′2 (20 μm)5 × 10−3 8 m / min:1 h / 88 20H / 250mol / l1 pass.F...
example 3
The effect of (a) added in an organic matrix based on cycloaliphatic epoxides (M1), polyol (T1) and in the presence of a levelling agent (BYK 306) is also evaluated.
In this example, the photoinitiator is an iodonium borate of formula (P2) added as a 33% w / w solution in isopropanol:
The following formulations were evaluated using RAPRA (cf. Table III and Table IV) for 0.5% and 1% of photoinitiator, respectively.
TABLE IIIReferenceF4F5F6M1 %82.766.157.45T1 %14.711.610.40Byk 306%1.10.80.65P2 %0.50.50.5a) in %02030Isopropanol %1.01.01Rapra (V.N.C) dose 0.4 J / cm2 T95 s690510420
TABLE IVReferenceF7F8F9M1 %81.464.856.3T1 %14.711.410.1Byk 306%1.10.80.6P2 %111a) in %02030Isopropanol %222Rapra (V.N.C) dose 0.4 J / cm2 T95 s180150130
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity ηr | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| viscosity ηr | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com