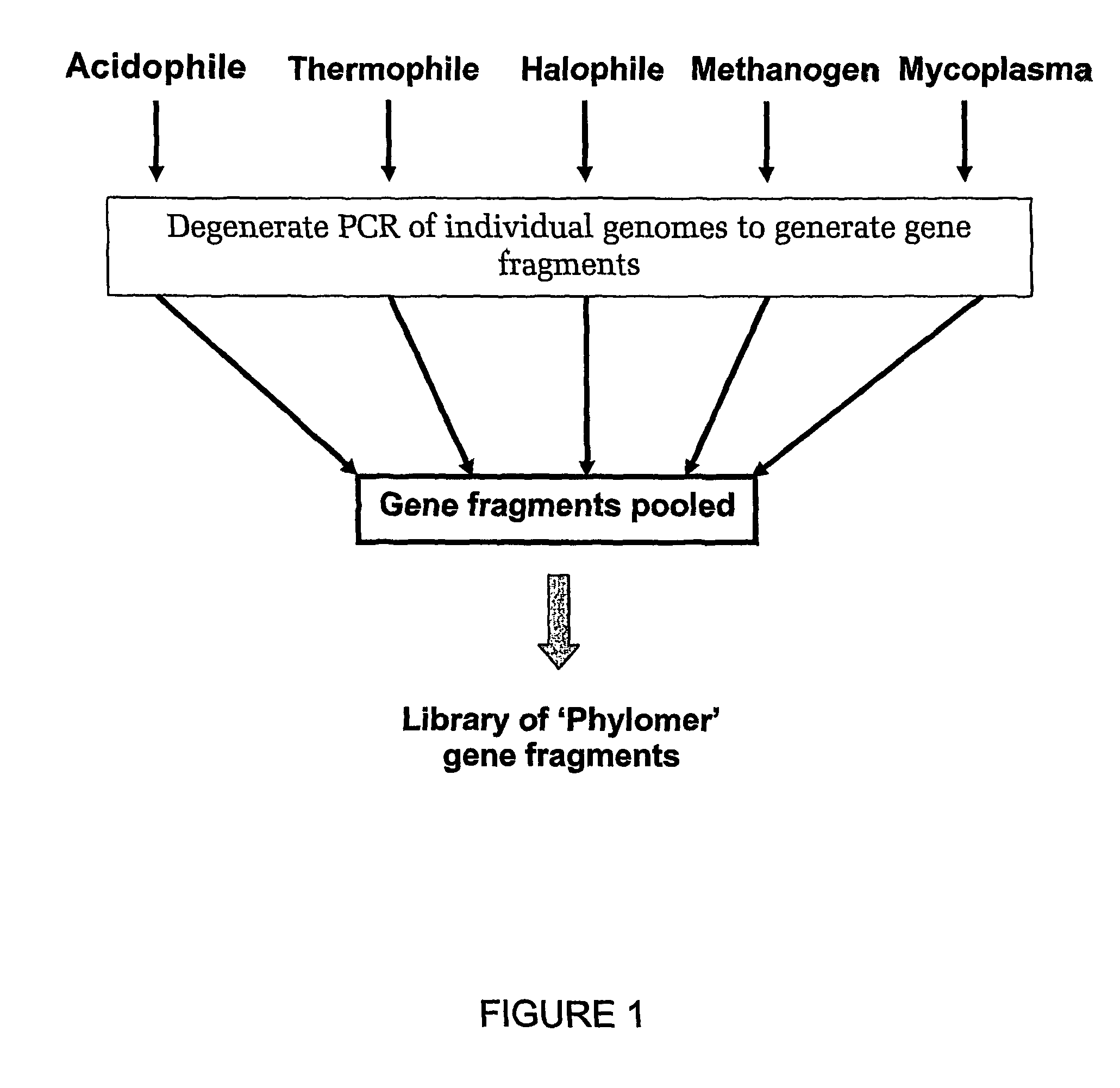
Methods of constructing biodiverse gene fragment libraries and biological modulators isolated therefrom
a technology of biological modulators and fragment libraries, which is applied in the field of nucleic acid fragment library production and production, can solve the problems of reducing the appeal of peptides as therapeutic agents, peptides often show little or none of the secondary or tertiary structure required for efficient binding, and their more rapid degradation and clearance, so as to enhance the nucleotide sequence diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Construction of a Biodiverse Nucleic Acid Fragment Expression Library in the Vector pDEATH-Trp
[0588]Nucleic acid was isolated from the following bacterial species:
[0589]
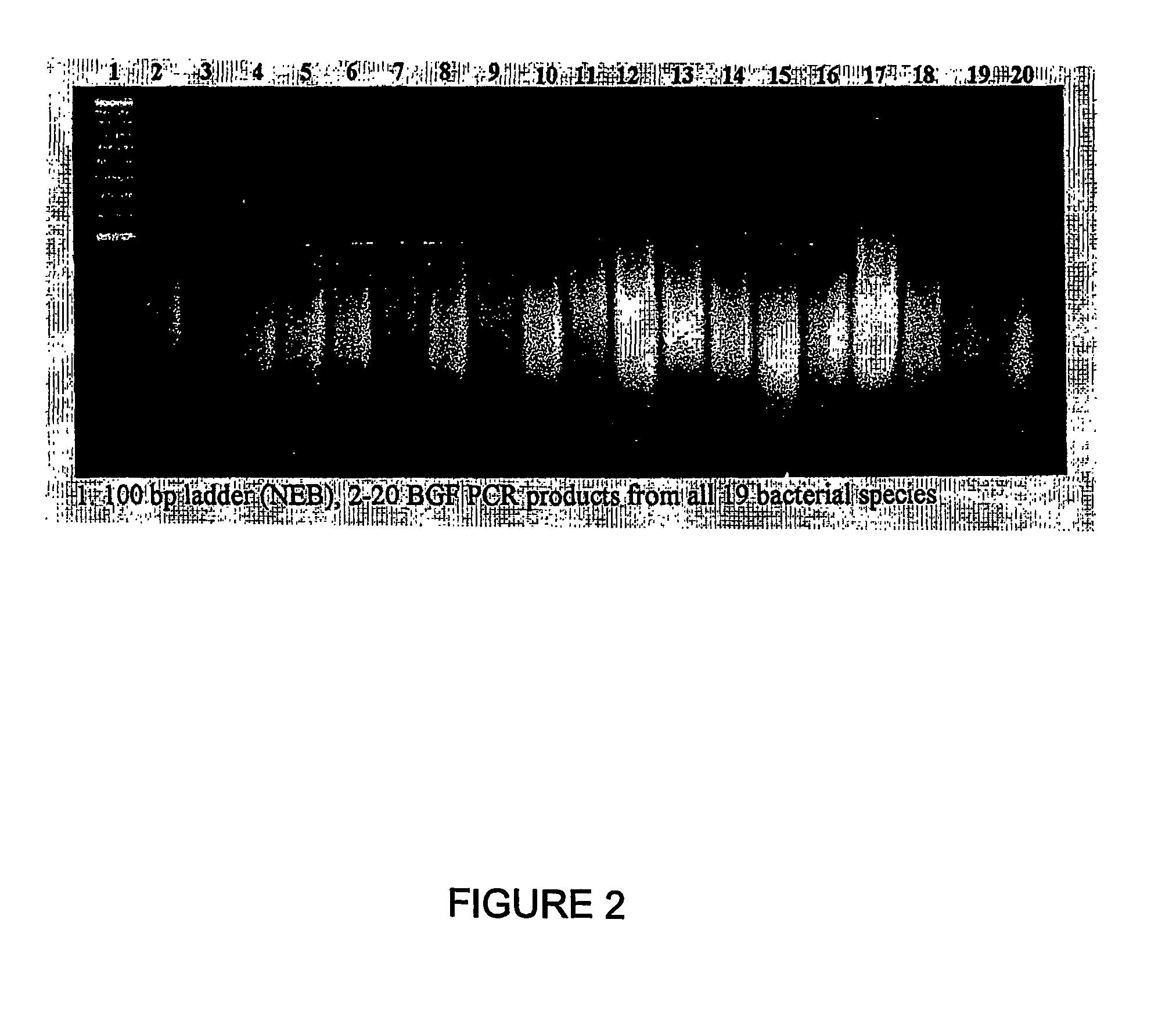
1Archaeoglobus fulgidis2Aquifex aeliticus3Aeropyrum pernix4Bacillus subtilis5Bordetella pertussis TOX66Borrelia burgdorferi7Chlamydia trachomatis8Escherichia coli Kl29Haemophilus influenzae (rd)10Helicobacter pylori11Methanobacterium thermoautotrophicum12Methanococcus jannaschii13Mycoplasma pneumoniae14Neisseria meningitidis15Pseudomonas aeruginosa16Pyrococcus horikoshii17S nechosistis PCC 680318Thermoplasma volcanium19Thermotoga maritima
[0590]Nucleic acid fragments were generated from the genomic DNA of each genome using 2 consecutive rounds of primer extension amplification using tagged random oligonucleotides with the sequence:
[0591]5′-GACTACAAGGACGACGACGACAAGGCTTATCAATCAATCAN6-3′ (SEQ ID NO: 33). The PCR amplification was completed using the Klenow fragment of E. coli DNA polymerase I in the following primer ...
example 2
Characterization of a Biodiverse Nucleic Acid Fragment Expression Library in the pDEATH-Trp Vector
[0627]Sequence analysis of nucleic acids cloned into pDEATH-Trp vector show that the fragments are derived from a variety of organisms, and encode a variety of proteins, as shown in Table 2.
[0628]
TABLE 2Characterization of nucleic acid fragment cloned into pDEATH-TrpInsert sizeGenbankNo.(bp)OrganismIDFunction1114P. aeruginosaAAG05339.1Hypothetical Protein2143SynechocystisBAA10184.1FructosePCC68033166E. coliAAC73742.1Lipoprotein4180B. subtilisCAB12555.1methyl-acceptingchemotaxisprotein5150N. meningitisAAF41991.1N utilizationsubstance protein A6240E. coliAAC75637.1Hypothetical protein7357H. pyloriAAD08555.1transcriptiontermination factorNusA883Z. maritimaAAD36283.1Hypothetical protein
example 3
Screening of a Biodiverse Nucleic Acid Fragment Library for Inhibitors of the Interaction Between the Polymyositis-Scleroderma Autoantigen (SCL) and Basic Helix-Loop-Helix Transcription Factor E47
[0629]Nucleic acid encoding the SCL protein was cloned into the prey vector pJFK (SEQ ID NO: 60; FIG. 5) in operable connection with a nuclear localisation signal, and a B42 activation domain. The nucleic acid encoding the E47 protein was cloned into the bait vector pDD (SEQ NO: 61; FIG. 6) in operable connection with the LexA DNA binding domain. The pDD vector also contains a nucleic acid encoding the HIS3 gene (FIG. 6).
[0630]These vectors were transformed into the PRT 480 yeast strain (which contains two LexA-CYH2 chimeric reporter genes and two LexA-URA3 counter selectable reporter genes).
[0631]The process of screening the library is represented schematically in FIG. 7. Briefly, the PRT 480-SCL / E47 bait prey haploid strain was grown to high density in complete synthetic media lacking his...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com