Optimized DNA and protein sequence of an antibody to improve quality and yield of bacterially expressed antibody fusion proteins
a technology of bacterially expressed antibodies and protein sequences, applied in the direction of antiinfectives, peptides, drug compositions, etc., can solve the problems of induction of undesired immune responses, adverse events in patients, and dramatically increased production costs, so as to achieve dramatic increases in production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Molecular Cloning of Modified Expression Constructs
Method
Construction of scFv(FRP5)-ETA Expression Vectors
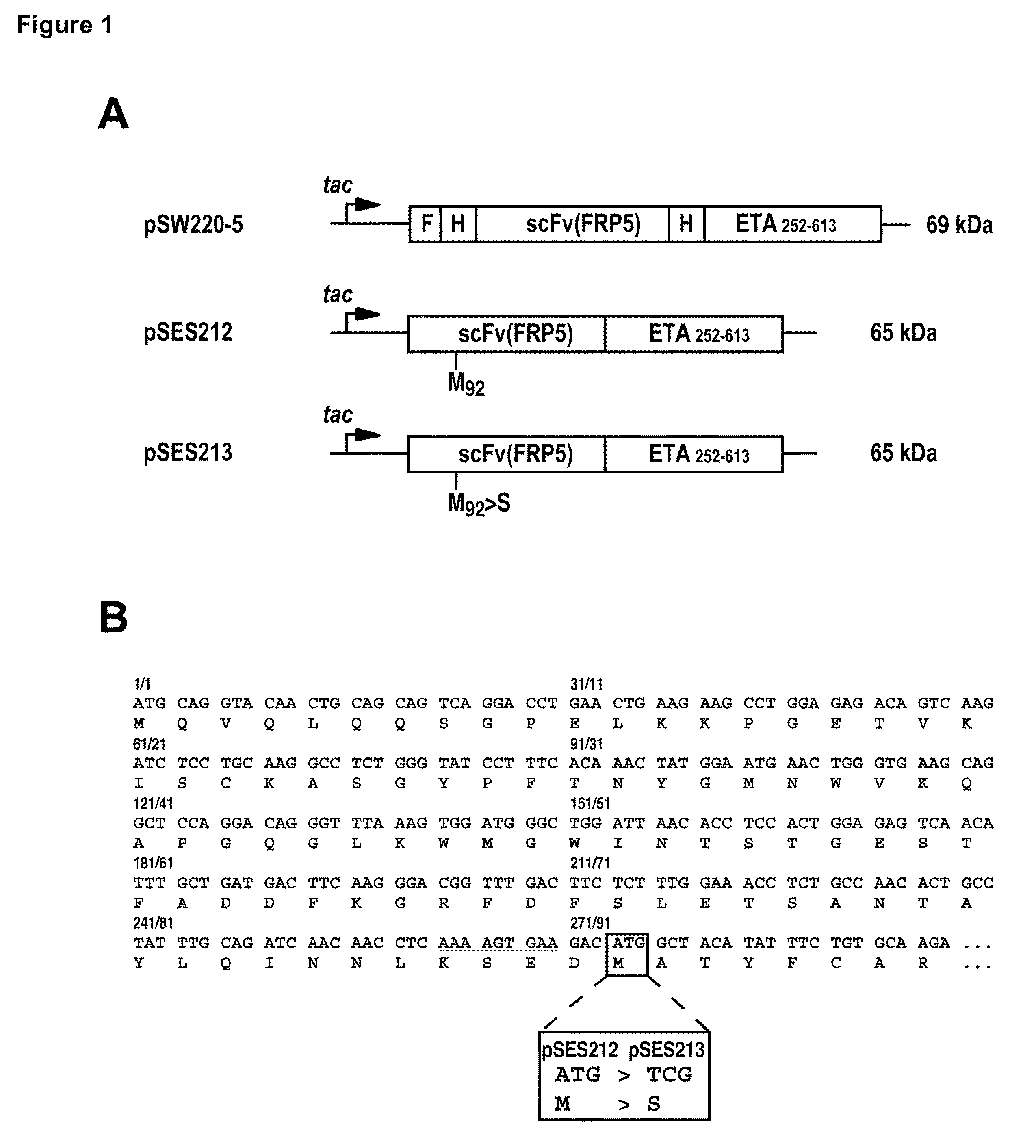
[0071]The plasmid pSW220-5 was described before (7). It contains sequences coding for an N-terminal FLAG tag, a first His6 cluster, the ErbB2-specific scFv(FRP5), a second His6 cluster, and truncated Pseudomonas exotoxin A (residues 252-613 of the wildtype toxin) in a single open reading frame. Plasmid pSES211 (unpublished; provided by TopoTarget Germany AG) contains an open reading frame for scFv(FRP5)-ETA originally derived from pSW220-5 and still including the first N-terminal His6 cluster, but lacking the N-terminal FLAG tag and the internal His6 cluster between scFv(FRP5) and exotoxin A sequences. Plasmid pSES212 was derived by deleting the remaining N-terminal His6 cluster of pSES211. The plasmid was generated by PCR using the oligonucleotide primers 5′NdeI-scFv(FRP5) 5′-CGATTAGCATATGCAGGTACAACTGCAGCAGTCAGGACC-3′ (SEQ ID NO:5) and 3′XbaI-scFv(FRP5) 5′-GCTGCCGCCCTCTAGAGCTTT...
example 2
Expression of scFv(FRP5)-ETA Derivatives in E. coli
Method
Expression of scFv(FRP5)-ETA Derivatives in E. coli, Preparation of Inclusion Bodies, Solubilization and Refolding
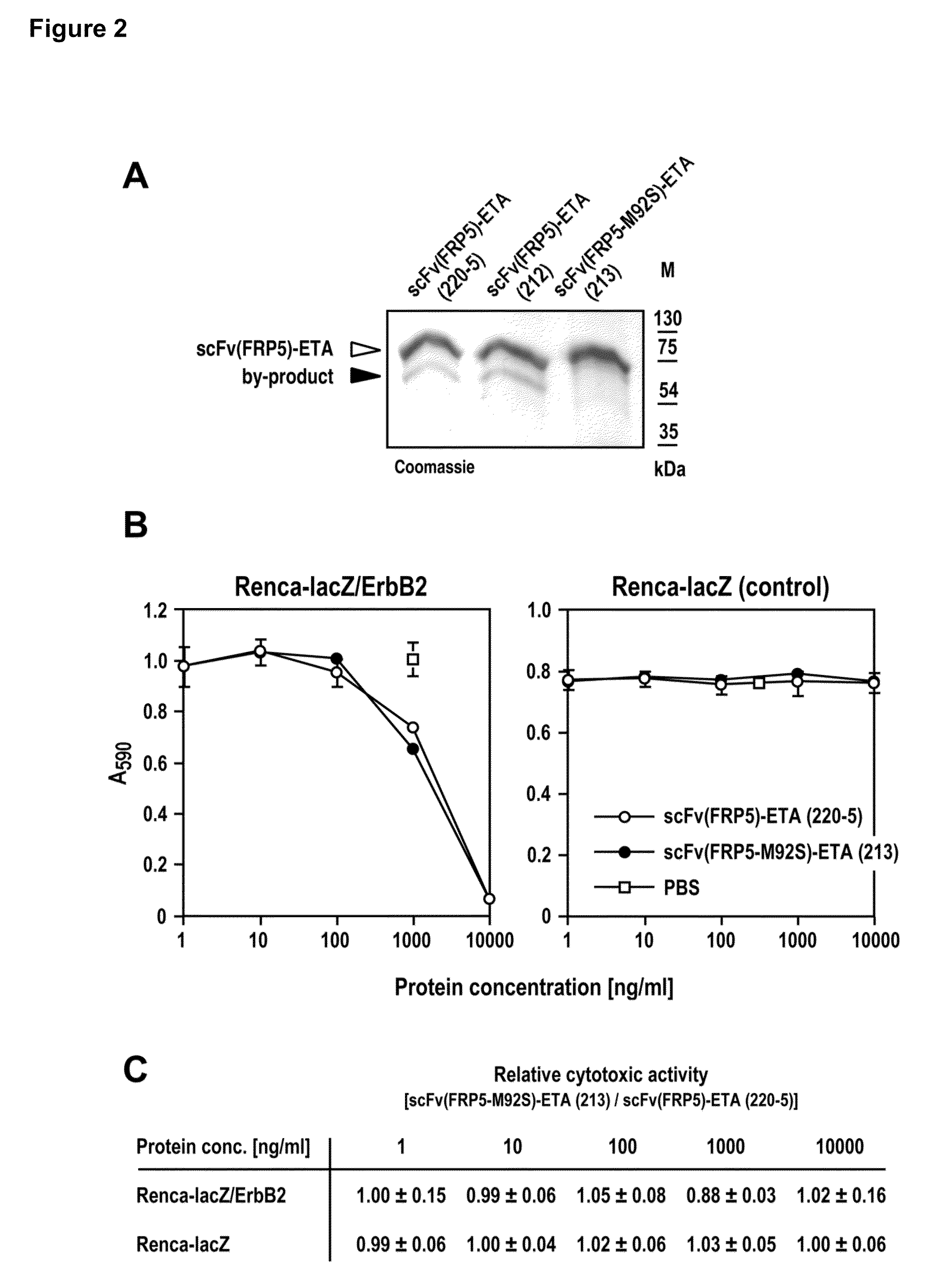
[0075]E. coli DH5α were transformed with the expression plasmids pSW220-5, pSES212 or pSES213. One liter expression cultures (LB, 0.5% glucose, 50 μg / ml kanamycin in the case of pSES212 or pSES213, or 100 μg / ml ampicillin in the case of pSW220-5) were grown at 37° C. to an OD600 of 0.8. The cultures were induced by addition of 0.5 mM IPTG for 3 hours. Cells were harvested by centrifugation (7500 g, 10 min), resuspended in PBS, and lysed in a French pressure cell. Inclusion bodies were collected by centrifugation (10000 g, 10 min, 4° C.) and washed by resuspension in washing buffer (2 M urea, 2% Triton X-100, 500 mM NaCl in PBS, pH 8) and subsequent centrifugation (10000 g, 10 min, 4° C.). Purified inclusion bodies were resuspended in solubilization buffer (8 M urea, 500 mM NaCl in PBS, pH 8). After centrifugation,...
example 3
Biological Activity of scFv(FRP5-M92S)-ETA
Method
Cells and Culture Conditions
[0078]Murine renal carcinoma cells stably expressing E. coli β-galactosidase (Renca-lacZ), or β-galactosidase and human ErbB2 (Renca-lacZ / ErbB2) (8) were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 units / ml penicillin, 100 μg / ml streptomycin, 0.25 mg / ml Zeocin (Invitrogen, Karlsruhe, Germany), and 0.48 mg / ml G418 (Renca-lacZ / ErbB2).
[0079]Cells were seeded in 96-well plates at a density of 1.5×104 cells / well in normal growth medium. Different concentrations of scFv(FRP5)-ETA fusion proteins or diluent were added to triplicate samples, and the cells were incubated for 48 h at 37° C. in 5% CO2 and 95% humidified air. An aliquot of 10 μl of 10 mg / ml MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma, Deisenhofen, Germany) in PBS was added to each well, and the cells were incubated for another 3 h. Cells were lysed by t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com